
Class 12 Chemistry Chapter 6 Question Answer - Haloalkanes and Haloarenes
NCERT Solutions for Class 12 Chemistry Chapter 6 – Haloalkanes and Haloarenes provided on this page are strictly designed as per the latest CBSE syllabus and NCERT guidelines. These solutions cover all textbook exercise questions along with important conceptual problems to help students understand the chapter thoroughly and score well in board examinations.
 Table of Content
Table of ContentAll the NCERT Solutions for Class 12 Chemistry are prepared by experienced subject experts using clear explanations, step-by-step answers, and proper chemical reasoning. Special care has been taken to present solutions in simple and easy-to-understand language, making them suitable for self-study and quick revision.
Chapter 6, Haloalkanes and Haloarenes, is available here in a well-structured PDF format that can be downloaded free of cost. Students can access these solutions anytime and anywhere to revise key concepts, practice numerical and theoretical questions, and strengthen their exam preparation.
Quick Insights of Class 12 Haloalkanes and Haloarenes NCERT Solutions
NCERT Solutions for Class 12 Chemistry Chapter 6 will give you insights into the General Introduction: The organic part of Haloalkanes gives the details of depth of Nomenclature, nature of the C–X bond, physical and chemical properties, and optical rotation mechanism of substitution reactions.
The section will give you crisp learnings on Haloarenes: Nature of the C–X bond, substitution reactions (Directive influence of halogen in monosubstituted compounds only).
The understanding related to topics like Uses and environmental effects of - dichloromethane, trichloromethane, tetrachloromethane, iodoform, freons, and DDT.
Using these solutions can help students analyse their level of preparation and understanding of concepts.
Class 12 Haloalkanes and Haloarenes NCERT solutions topics are included according to the revised academic year 2025-26 syllabus.
It also provides resources such as class notes, important concepts, and formulas exemplar solutions.
Class 12 Chemistry Chapter 6 Question Answer Practice
1. Write structures of the following compounds:
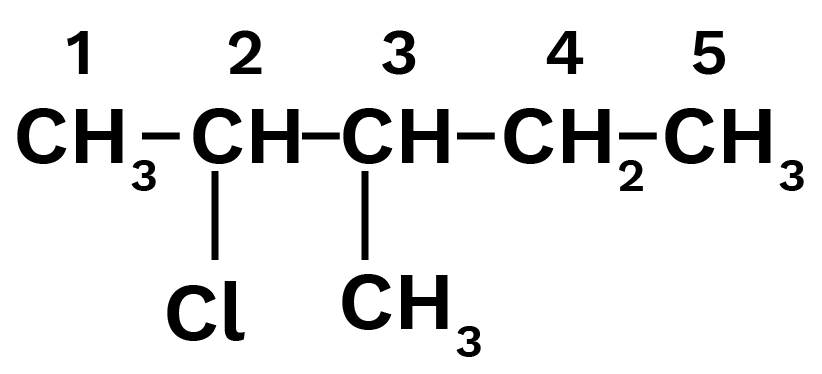
2 - chloro-3methylpentane:
Ans: Main chain will be of five carbon atoms. At second position chlorine will be present and at third position, methyl group will be present.
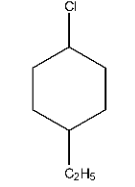
1- chloro- 4 – ethylcyclohexane
Ans: A cyclic structure is present in which at 1st carbon there is chlorine present and at the 4th position ethyl group is present.
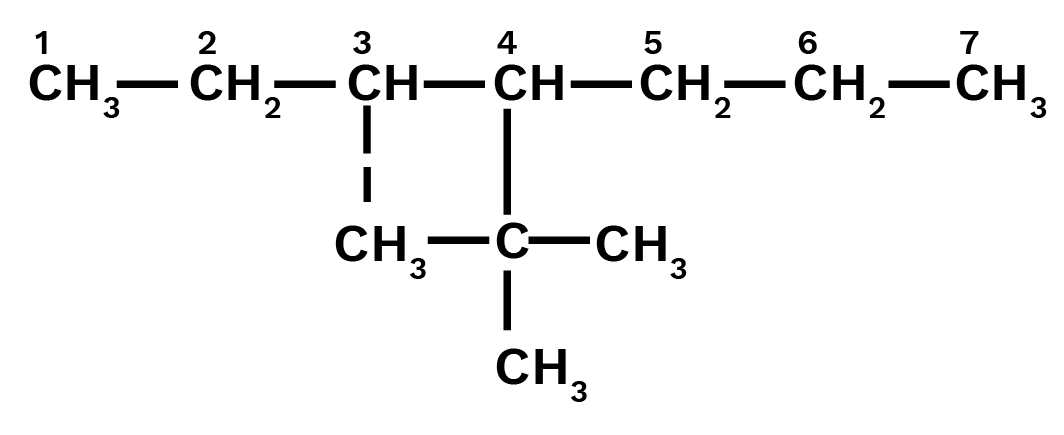
4 - tert .Butyl-3- iodoheptane
Ans: Main chain will be of seven carbon atoms. At 4th position tertiary butyl will be present and at the 3rd position iodine will be present.
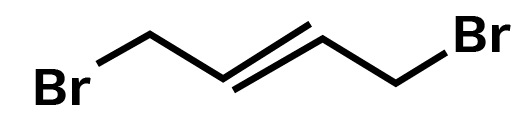
1, 4 -Dibromobut- 2 – ene
Ans: Main chain will be of 4 carbon atoms and it is an alkene. There are two bromine atoms on the terminal carbon atoms.
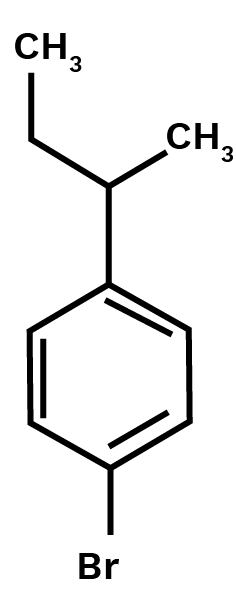
1-Bromo- 4 - sec/ butyl- 2 -methylbenzene.
Ans: Main chain will be the benzene. Three substituents are present on benzene.
2. Why is sulphuric acid not used during the reaction of alcohols with $\text{KI}$?
Ans: ${{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}$ is a powerful oxidizer. $\text{HI}$generated during the reaction is oxidized, preventing the interaction between an alcohol and $\text{HI}$ from becoming alkyl iodide as a result of the process. ${{\text{H}}_{\text{3}}}\text{P}{{\text{O}}_{\text{3}}}$ is utilized as a non-oxidizing acid to prevent the oxidation of $\text{HI}$. The reaction is given below:
\[\text{2KI + }{{\text{H}}_{\text{2}}}\text{S}{{\text{O}}_{\text{4}}}\to \text{ 2KHS}{{\text{O}}_{\text{4}}}\text{+ 2HI }\to \text{ }{{\text{I}}_{\text{2}}}\]
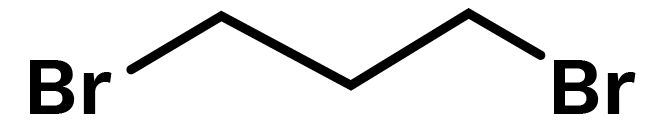
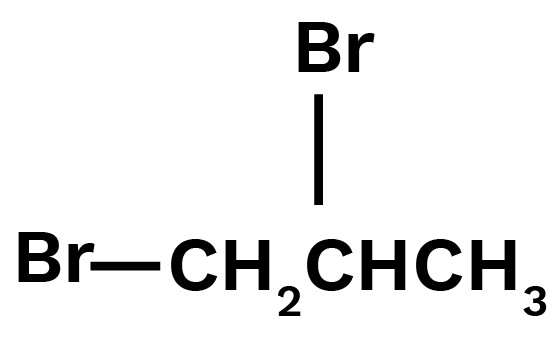
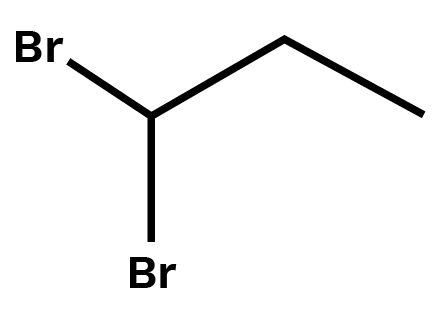
3. Write structures of different dihalogen derivatives of propane.
Ans: Propane is an alkane molecule containing three carbon atoms. Dihalogen derivatives are propane that includes two halogen atoms. There are four potential isomers. These are given below:
1,3-Dibromopropane
1,2-Dibromopropane
1,1-Dibromopropane
2,2-Dibromopropane
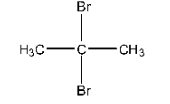
4. Among the isomeric alkanes of molecular formula ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}$ identify the one that one photochemical chlorination yields:
Ans: Isomeric alkane means that there will be different arrangements of substituents around the carbon atom. ${{\text{C}}_{\text{5}}}{{\text{H}}_{\text{12}}}$ is pentane and it is an alkane.
A Single Monochloride
Isomer of pentane that will yield single monochloride is neopentane and its structure is given below:
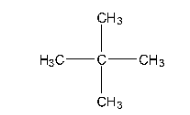
Due to the fact that all of the H-atoms are identical, replacing any one of them will result in the same result.
Three isomeric monochlorides.
Isomer of pentane that will yield three isomeric monochlorides is n-pentane as given below:
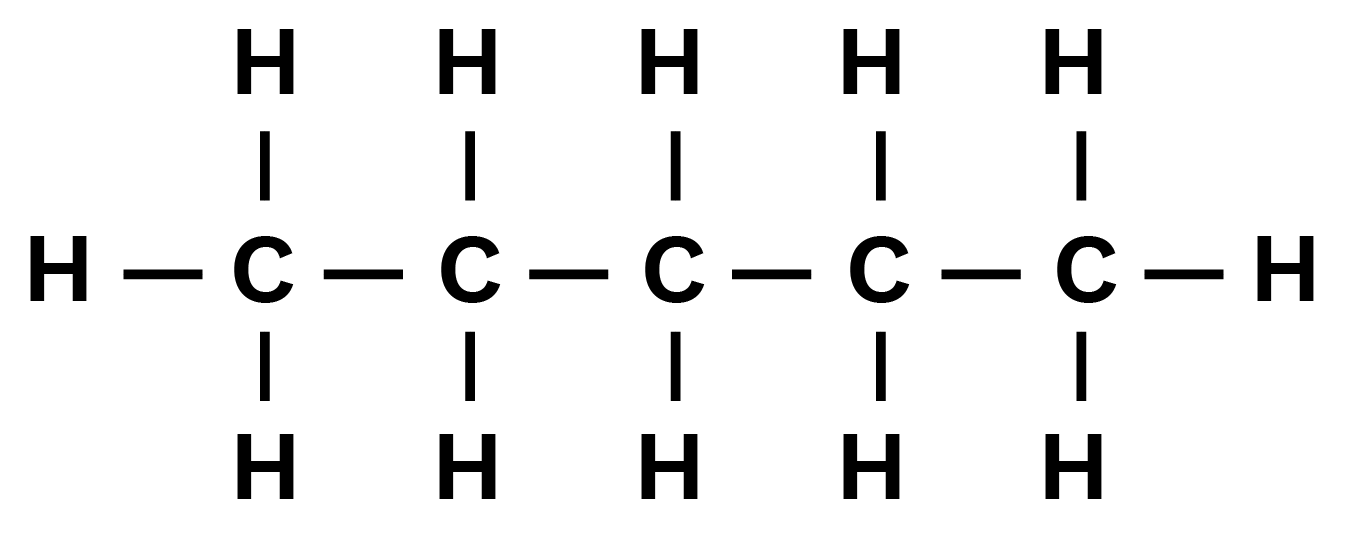
In this a, b, and c have three sets of equivalent hydrogen atoms.
Four isomeric monochlorides.
Isomer of pentane that will yield four isomeric monochlorides is iso-pentane which is given below:
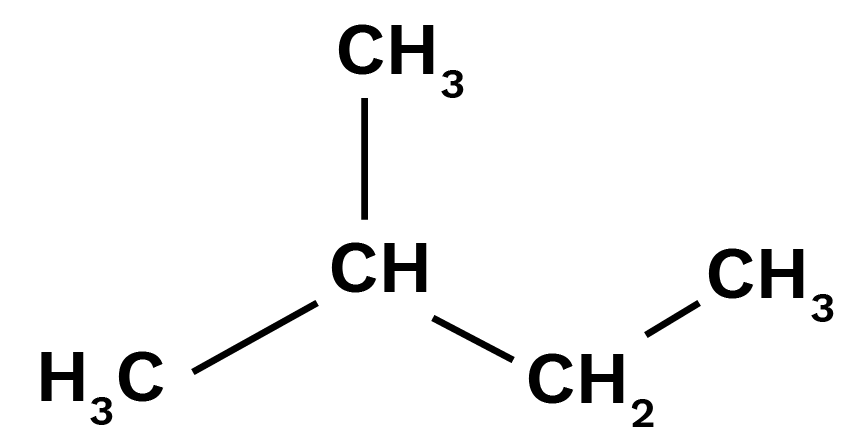
Because all the carbon atoms have different hydrogen atoms.
5. Draw the structures of major monohalo products in each of the following reactions:
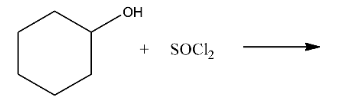
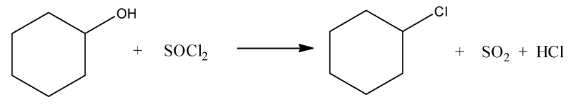
Ans: In this reaction the hydroxyl group will be replaced with the chlorine atom. The reaction is given below:
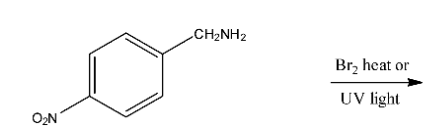
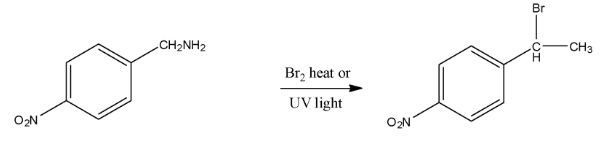
Ans: In this reaction the amine group will be replaced with methyl bromide. The reaction is given below:
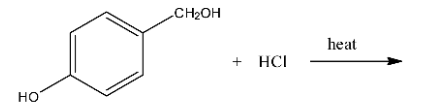
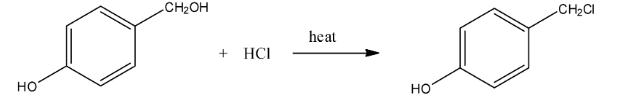
Ans: In this reaction the hydroxyl group with the methyl group will be replaced with the chlorine atom. The reaction is given below:
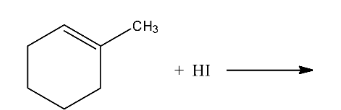
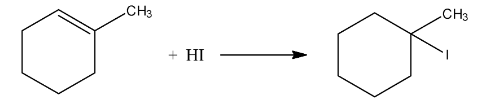
Ans: In this reaction the iodine from the hydrogen iodide will attach the carbon atom having the double bond as well as methyl group. The reaction is given below:
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{Br+NaI}\to $
Ans: In this reaction the iodine atom will replace the bromine in the haloalkane. The reaction is given below:
\[\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{Br + NaI }\to \text{ C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{I + NaBr}\]
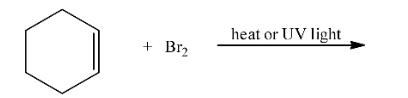
Ans: In this reaction, the bromine atom will attack the alpha-carbon atom of the double bond. The reaction is given below:

6. Arrange each set of compounds in order of increasing boiling points.
Bromomethane, Bromoform, Chloromethane, Dibromomethane.
Ans: As we can see that all the compounds given above are haloalkanes. The order will be:
Chloromethane < Bromomethane < Dibromomethane < Bromoform
This is due to the fact that as the halogen size increases the boiling point will increase and as the number of halogen atoms increases in the same chain, the boiling point will increase.
Chloropropane, Isopropyl chloride, 1 -Chlorobutane.
Ans: In all the compounds there is chlorine atom present and the size of the alkyl chain is different. The order will be:
Isopropyl chloride < 1- Chloropropane < 1 – Chlorobutane
This is due to the fact that as the branching of the chain increases the boiling point will decrease and as the size of the chain increases the boiling point will increase.
7. Which alkyl halide from the following pairs would you expect to react more rapidly by an ${{\text{S}}_{\text{N}}}\text{2}$ mechanism? Explain your answer?
Ans: For the ${{\text{S}}_{\text{N}}}\text{2}$ reaction the order of reactivity is ${{1}^{\circ }}>{{2}^{\circ }}>{{3}^{\circ }}$, so we can solve the question according to the order of the reactivity.
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{Br}$ or
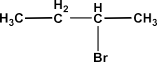
Ans: In the first compound the alkyl halide is primary halide while the second compound is a secondary halide. So, the first compound will react more rapidly.
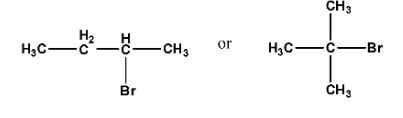
Ans: In the first compound the alkyl halide is secondary halide while the second compound is a tertiary halide. So, the first compound will react more rapidly.
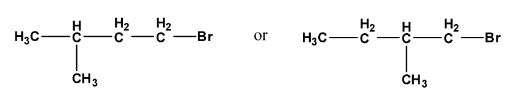
Ans: Both compounds are secondary halides but the steric hindrance by the $\text{C}{{\text{H}}_{\text{3}}}$ group will have more effect in the second compound so, the first compound will react more rapidly.
8. In the following pairs of halogen compounds, which compound undergoes faster ${{\text{S}}_{\text{N}}}\text{1}$ reaction?
Ans: For the ${{\text{S}}_{\text{N}}}1$reaction the order of reactivity is ${{3}^{\circ }}>{{2}^{\circ }}>{{1}^{\circ }}$, so we can solve the question according to the order of the reactivity.
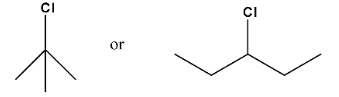
Ans: The first compound is tertiary compound and the second compound is secondary compound. So, the first compound will undergo a faster ${{\text{S}}_{\text{N}}}1$reaction.
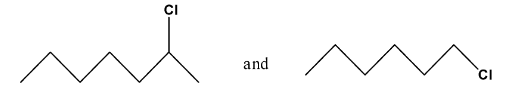
Ans: The first compound is a secondary compound and the second compound is primary compound. So, the first compound will undergo a faster ${{\text{S}}_{\text{N}}}1$reaction.
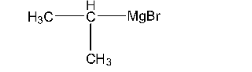
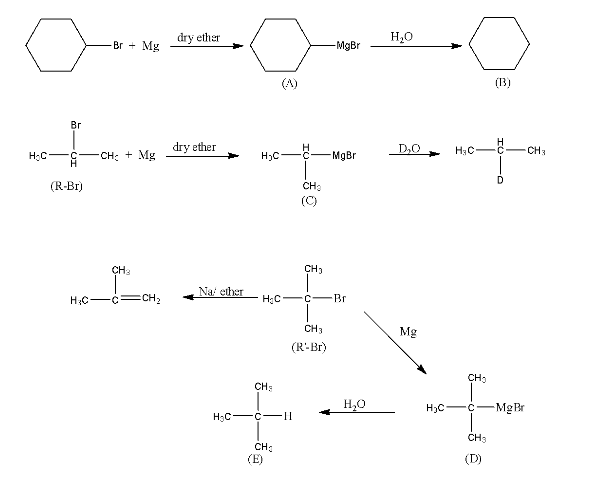
9. Identify A, B, C, D, E, R and ${{\text{R}}^{\text{1}}}$ in the following:
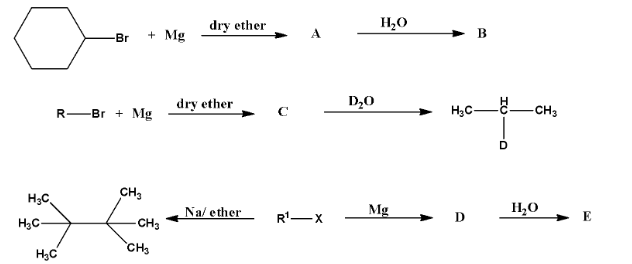
Ans: The compound A in the reaction is cyclohexylmagnesium bromide, compound B is Cyclohexane.
Compound R –Br will be 2-Bromopropane and the compound C is given below:
The third part of the question is incorrect because the tertiary-alkyl halides do not undergo wurtz reaction but they undergo dehydrohalogenation to give alkenes.
So, the compound ${{\text{R}}^{\text{1}}}\text{-Br}$ is given below:
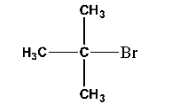
Compound D is Tertiary butyl magnesium bromide and compound E is 2-Methylpropane.
The complete reaction is given below:
NCERT Exercise
1. Name the following halides according to IUPAC system and classify them as alkyl, benzyl (primary, secondary, tertiary), vinyl or aryl halides:
${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{2}}}\text{CHCH(Cl)C}{{\text{H}}_{\text{3}}}$
Ans: The name of the compound is 2-Chloro-3-methyl butane and it is a secondary alkyl halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)CH(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{)Cl}$
Ans: The name of the compound is 3-Chloro-4-methyl hexane and it is a secondary alkyl halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{C}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{I}$
Ans: The name of the compound is 1-Iodo-2,2-dimethyl butane and it is a primary alkyl halide.
${{\text{(C}{{\text{H}}_{\text{3}}}\text{)}}_{\text{3}}}\text{CC}{{\text{H}}_{\text{2}}}\text{CH(Br)}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{5}}}$
Ans: The name of the compound is 1-Bromo-3,3-dimethyl-1-phenylbutane and it is a secondary benzylic halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)CH(Br)C}{{\text{H}}_{\text{3}}}$
Ans: The name of the compound is 2-bromo-3-methyl butane and it is a secondary alkyl halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{C(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}{{\text{)}}_{\text{2}}}\text{C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}$
Ans: The name of the compound is 1-bromo-2-ethyl-2-methyl butane and it is a primary alkyl halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{C(Cl)(}{{\text{C}}_{\text{2}}}{{\text{H}}_{\text{5}}}\text{)C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}$
Ans: The name of the compound is 3-Chloro-3-methyl pentane and it is a tertiary alkyl halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{CH=C(Cl)C}{{\text{H}}_{\text{2}}}\text{CH(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
Ans: The name of the compound is 3-Chloro-5-methyl hex-2-ene and it is a vinylic halide.
$\text{C}{{\text{H}}_{\text{3}}}\text{CH=CHC(Br)(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
Ans: The name of the compound is 4-bromo-4-methyl pent-2-ene and it is allylic halide.
$\text{p-Cl}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{C}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{2}}}$
Ans: The name of the compound is 1-Chloro-4-(2-methylpropyl) benzene and it is an aryl halide.
$\text{m-ClC}{{\text{H}}_{\text{2}}}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{C}{{\text{H}}_{\text{2}}}\text{C(C}{{\text{H}}_{\text{3}}}{{\text{)}}_{\text{3}}}$
Ans: The name of the compound is 1-Chloromethyl-3-(2,2-dimethylpropyl) benzene and it is a primary benzylic halide.
$\text{o-Br-}{{\text{C}}_{\text{6}}}{{\text{H}}_{\text{4}}}\text{CH(C}{{\text{H}}_{\text{3}}}\text{)C}{{\text{H}}_{\text{2}}}\text{C}{{\text{H}}_{\text{3}}}$
Ans: The name of the compound is 1-Bromo-2-(1-methylpropyl) benzene and it is an aryl halide.
2. Give the IUPAC names of the following compounds:
(i)CH3CH(Cl)CH(Br)CH3
Ans: The IUPAC name of this compound is 2-Bromo-3-chlorobutane.
(ii)CHF2CBrClF
Ans: The IUPAC name of this compound is 1-Bromo-1-chloro-1, 2, 2- trifluoroethane.
(iii)ClH2C≡CCH2Br
Ans: The IUPAC name of this compound is 1-Bromo-4-chlorbut-2-yne.
(iv)(CCl 3 ) 3 CCl
Ans: The IUPAC name of this compound is 2-(Trichloromethyl)- 1, 1, 1, 2, 3, 3, 3- heptachloropropane.
(v)CH3C(p-ClC6H4 )2 CH 3
Ans: The IUPAC name of this compound is 2-Bromo-3, 3-bis-(4-chlorophenyl) butane.
(vi)(CH3 )3CCH=C(Cl)C6 H4 I-p
Ans: The IUPAC name of this compound is 1-Chlor-1-(4-iodophenyl)-3, 3- dimethylbut-1-ene.
3. Write the structures of the following organic halogen compounds:
(i) 2-Chloro-3-methylpentane
Ans: The structure of this compound will be:
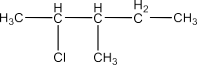
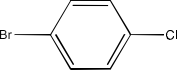
(ii) p-Bromochlorobenzene
Ans: The structure of this compound will be:
(iii) l-Chloro-4-ethylcyclohexane
Ans: The structure of the compound will be:
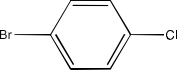
(iv) 2- (2-Chlorophenyl) -1- iodooctane
Ans: The structure of the compound will be:

(v) 2-Bromobutane
Ans: The structure of the compound will be:
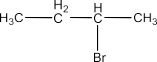
(vi) 4-tert-Butyl-3-iodoheptane
Ans: The structure of the compound will be:
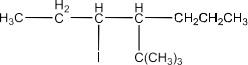
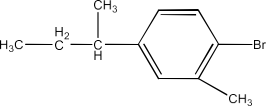
(vii) 1-Bromo-4-sec-butyl-2-methylbenzene
Ans: The structure of the compound will be:
(viii) l, 4-Dibromobut-2-ene
Ans: The structure of the compound will be:
BrCH2 -CH=CH-CH2 Br
4. Which one of the following has the highest dipole moment? CH2Cl2, CHCl3 or CCl4
Ans: Below are the three-dimensional structures of the three compounds, as well as the direction of each bond's dipole moment:
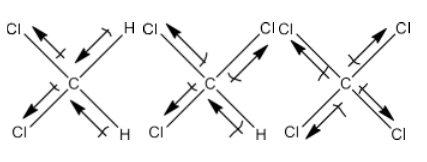
CCl4has no dipole moment since it is symmetrical. When two C-Cl dipole moments are added to CHCl3, the C-H and C-Cl bonds oppose each other. CHCl3has a limited dipole moment (1.03 D) because the dipole moment of the second resultant is anticipated to be less than that of the first. This means that in CH2Cl2, the resulting dipole moment of C-Cl pairs is greater than in CHCl3. Due to its dipole moment, CH2 Cl2 is the strongest.
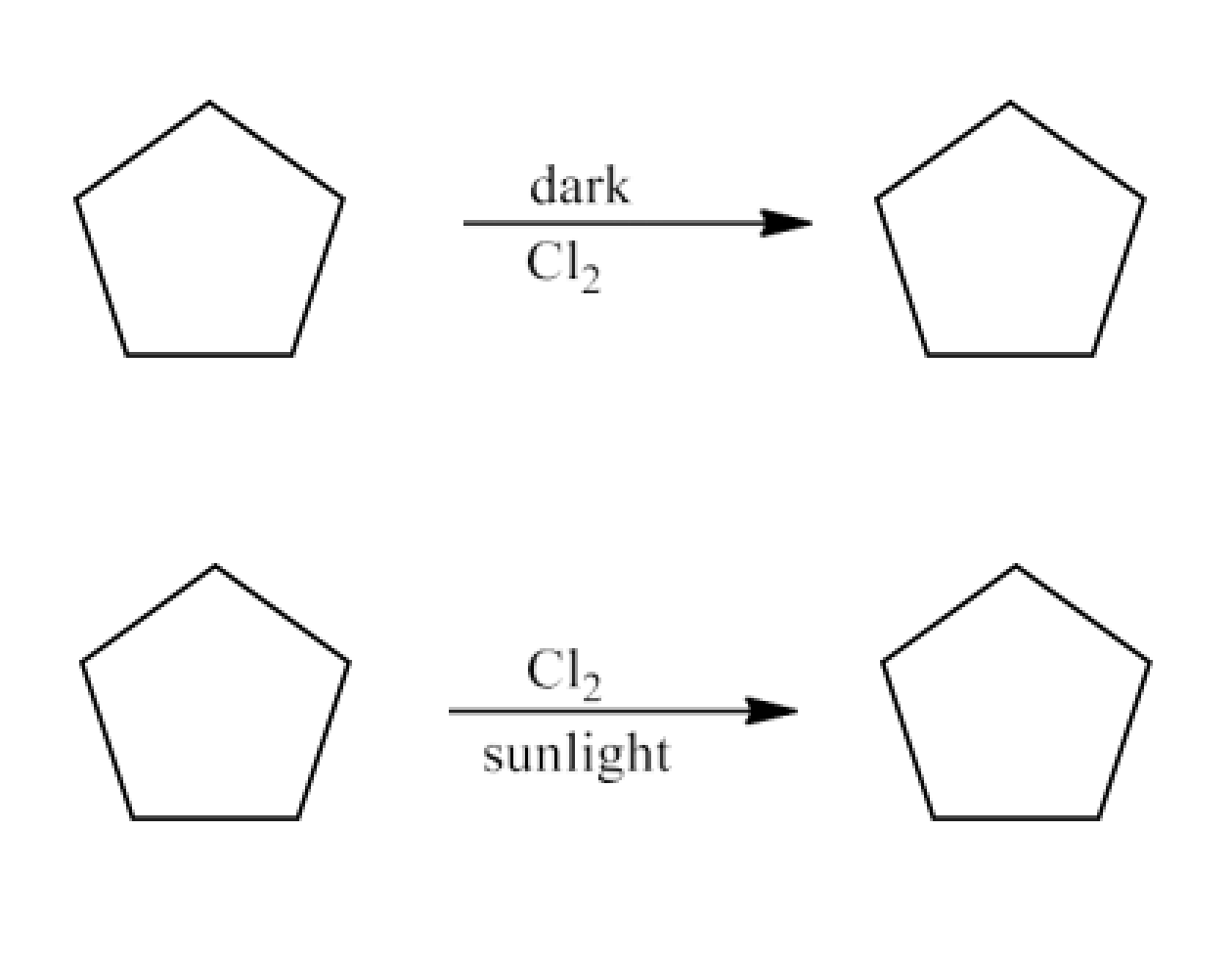
5. A hydrocarbon C5H10does not react with chlorine in dark but gives a single monochloro compound C5H9Cl in bright sunlight. Identify the hydrocarbon.
Ans: The molecular formula of the hydrocarbon might be either cycloalkane or alkene. Since the molecule does not react with Cl2in the dark, it must be a cycloalkane. Since the cycloalkane interacts with Cl2in the presence of strong sunshine to form a single monochloro compound, C5H9Cl, all ten hydrogen atoms in the cycloalkanes must be equivalent. Thus, cyclopentane is the cycloalkane.
6. Write the isomers of the compound having formula C4H9Br.
Ans: First we have find the Double bond equivalent (DBE) for C4H9Br. It is given below:
$\frac{4(4-2)+9(1-2)+1(1-2)}{2}+1=0$
The answer is zero which means that there is no ring or unsaturation in the isomers of the given compound, all the isomers will be either branched or straight chain. So, there will be four isomers and these are given below:
(i)1- Bromobutane:
CH3CH2CH2CH2Br
(ii) 1-Bromo-2-Methylpropane:
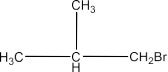
(iii) 2-Bromo Butane:
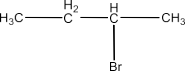
(iv) 2-Bromo-2-Methylpropane:
7. Write the equation for the preparation of 1-iodobutane from:
(i)1-Butanol
Ans: 1-Butanol will react with potassium iodide and phosphoric acid to form the 1- Iodobutane. The reaction is given below:
$CH_3CH_2CH_2CH_2OH+KI+H_3PO_4 \rightarrow CH_3CH_2CH_2CH_2I+H_2O+KH_2PO_4$
(ii) 1-Chlorobutane
Ans: 1-Chlorobutane will react with potassium iodide in the presence of acetone to form the 1-Iodobutane. The reaction is given below:
$CH_3CH_2CH_2CH_2Cl + KI \overset{Acetone}\rightarrow CH_3CH_2CH_2CH_2I + KCI$
(iii)But-1-ene
Ans: But-1-ene will react with hydrogen bromide in the presence of peroxide to give 1-Bromobutane and then it reacts with sodium iodide in the presence of acetone to give 1-Iodobutane. The reaction is given below:
$CH_3CH_2-CH+HBr\overset{Acetone}{\rightarrow}CH_3CH_2CH_2CH_2I+KCI$
8. What are ambident nucleophiles? Explain with an example.
Ans: Ambident nucleophiles are nucleophiles that can attack at two distinct locations. It is a resonance hybrid of the following two structures (for example, the cyanide ion).Cyanide ion is a resonance hybrid of the following two structures:
$\colon \overset{\mathbf-}C\equiv N\colon\longleftrightarrow\colon C = \overset{\mathbf-}N:$
It may attack carbon to produce cyanide, and nitrogen to form isocyanide, depending on the chemical.
9. Which compound in each of the following pairs will react faster in $S_n 2$ reaction with - OH?
(i)CH3 Br or CH3 I
Ans: Both the compounds are alkyl halide but the iodide ion is a larger atom than bromide ion. So, -Iion is better leaving group than - Brion. Therefore, CH I3will react faster than CH 3 Br towards $S_n 2$ reaction with hydroxyl ion.
(ii) (CH3 )3 CCl or CH 3 Cl
Ans: In SN2 reaction the steric hindrance should be very less. (CH3)3 CClhas very high steric hindrance and CH3Cl has less steric hindrance. So, CH3Cl will react faster to the SN 2reaction with hydroxyl ion.
10. Predict all the alkenes that would be formed by dehydrohalogenation of the following halides with sodium ethoxide in ethanol and identify the major alkene:
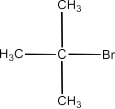
(i) 1-Bromo-1-methylcyclohexane
Ans: The major product when 1-Bromo-1-methylcyclohexane reacts with sodium ethoxide in ethanol will give 1-Methylcyclohexene. The reaction is given below:
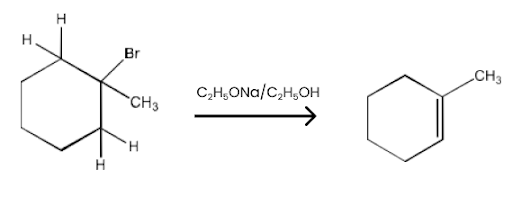
(ii) 2-Chloro-2-methylbutane.
Ans: 2-Chloro-2-methyl butane has two different sets of equivalent beta-hydrogen and hence, in principle, can give two alkenes. But according to Satzeff’s rule, more highly substituted alkene is more stable and is the major product. The reaction is given below:
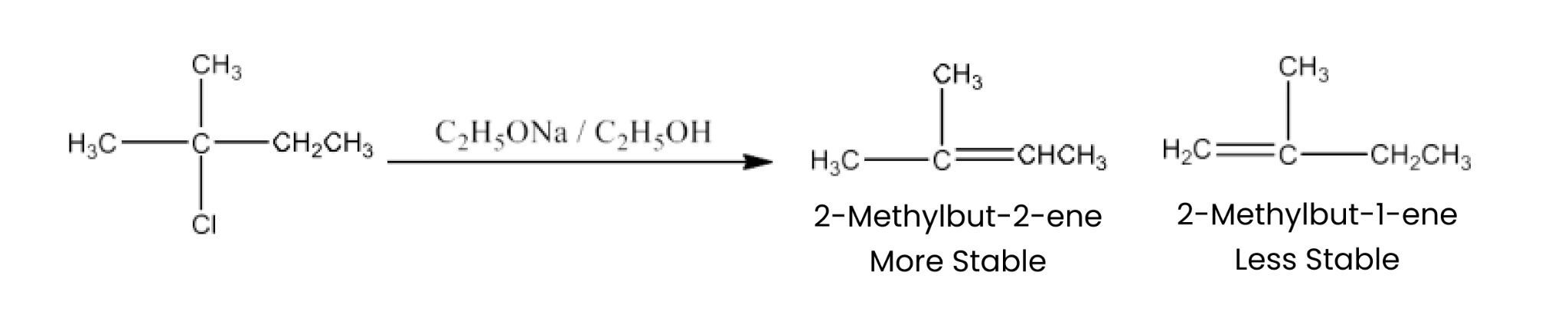
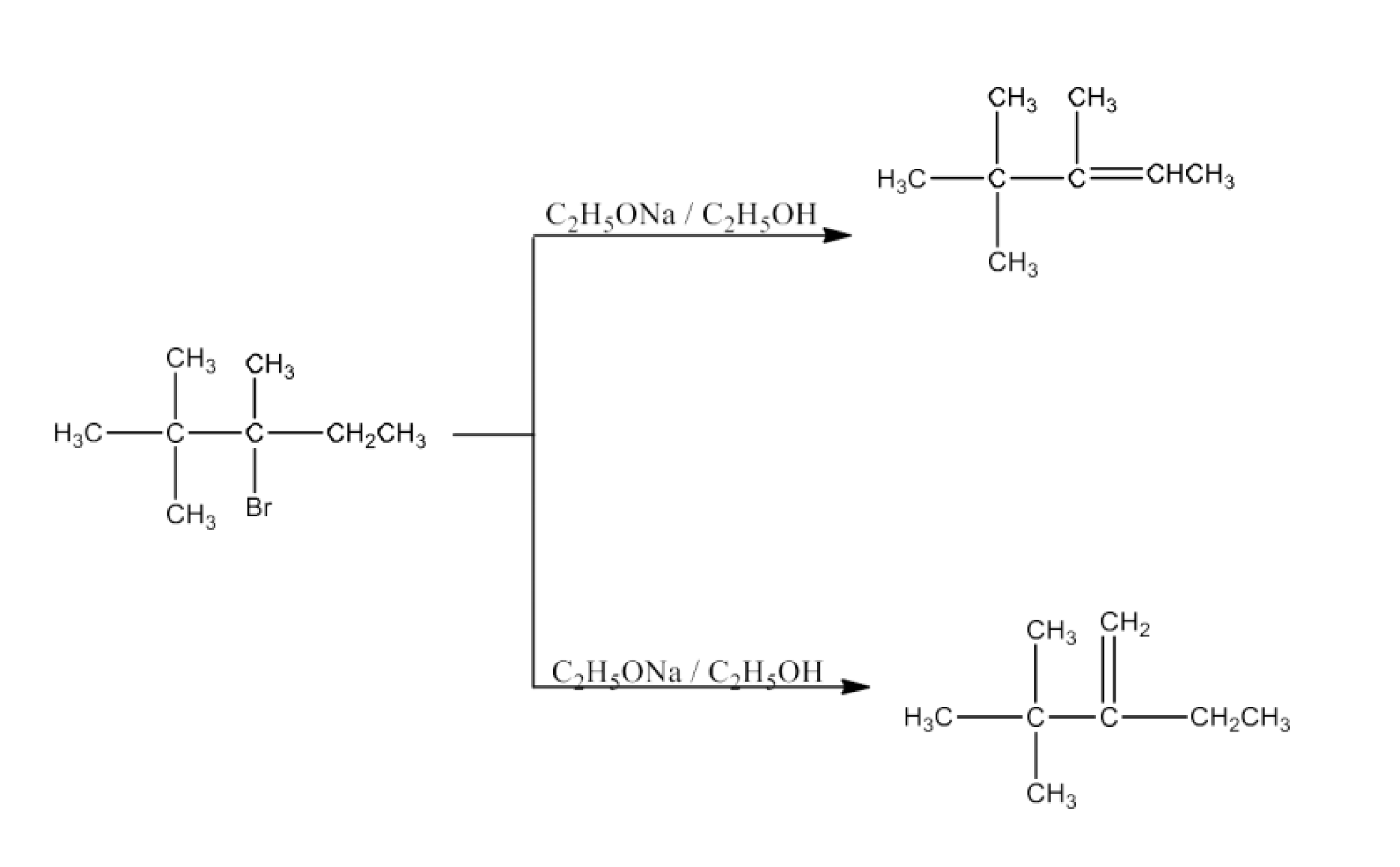
(iii) 2, 2, 3-Trimethyl-3-bromopentane.
Ans: 2, 2, 3-Triethyl-3-bromopentane has two different sets of equivalent beta hydrogen and hence, in principle, can give two alkenes. But according to Satzeff’s rule, more highly substituted alkene is more stable and is the major product. The reaction is given below:
11. How will you bring about the following conversions?
(i) Ethanol to but-1-yne.
Ans: Ethanol will react with SOCl2and pyridine to Chloroethane. Acetylene will react with NaNH2to form sodium acetylide. Now Chloroethane and Sodium acetylide will react to form But-1-yne. The reactions are given below:
$CH_3CH_20H\overset{SOCl_2,Pyridine }{\rightarrow}CH_3CH_2-CI$
$CH \equiv CH + NaNH_2 \overset{Liq. NH , 196K }{\rightarrow}HC \equiv C^{-}NA^+4$
$CH_3CH_2CI + HC \equiv C^- Na^+\rightarrow CH_3CH_2-C \equiv CH +NaCI$
(ii) Ethane to bromoethene
Ans: Ethane will react with bromine to form bromoethane. Now, this bromoethane will react with KOH to form ethene and then there will be the formation of 1, 2- Dibromoethane. Now, 1, 2-Dibromoethane will react with alcoholic KOH to form Bromoethene. The reactions are given below:
$CH_3-CH_3 + Br_2 \overset{hv, 520-670K }{\rightarrow}CH_3CH_2 Br\overset{ KOH(alc.) }{\rightarrow}CH_2=CH_2$
$CH_2=CH_2 \overset{Br_2/CCI_4 }{\rightarrow}Br CH_2=CH_2 Br \overset{KOH(alc.)}{\rightarrow}CH_2= CHBr$
(iii) Propene to 1-Nitropropane
Ans: Propene will react with Hydrogen bromide in peroxide effect to form 1- Bromopropane. Now, 1-Bromopropane will react with silver nitrite to given 1- Nitropropane. The reaction is given below:
$CH_3-CH=CH_2 \overset{HBr}{\rightarrow}CH_3CH_2CH_2Br \overset{AgNO_2}{\rightarrow}CH_3CH_2CH_2NO_2$
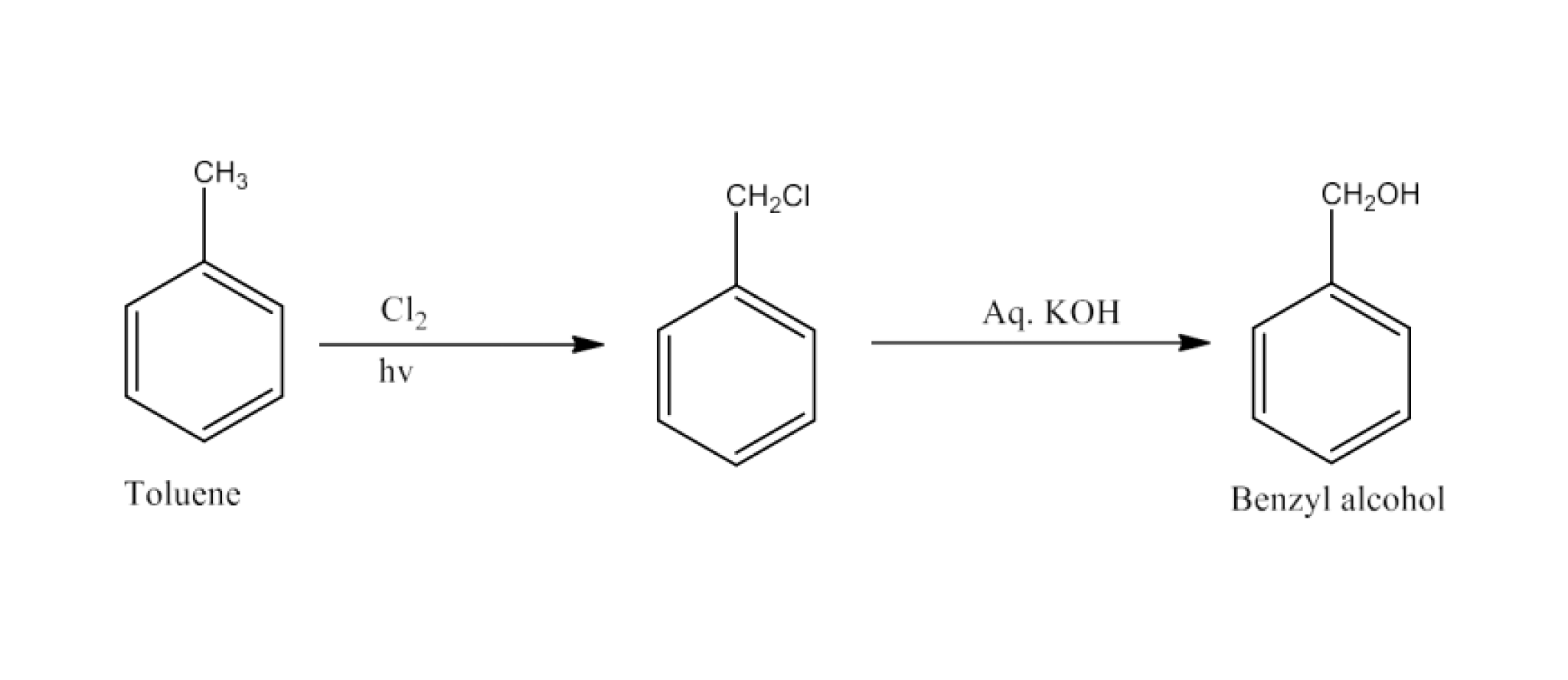
(iv) Toluene to benzyl alcohol
Ans: Toluene will react with chlorine in the presence of light to give benzyl chloride and this benzyl chloride will react with aqueous KOH to give benzyl alcohol. The reaction is given below:
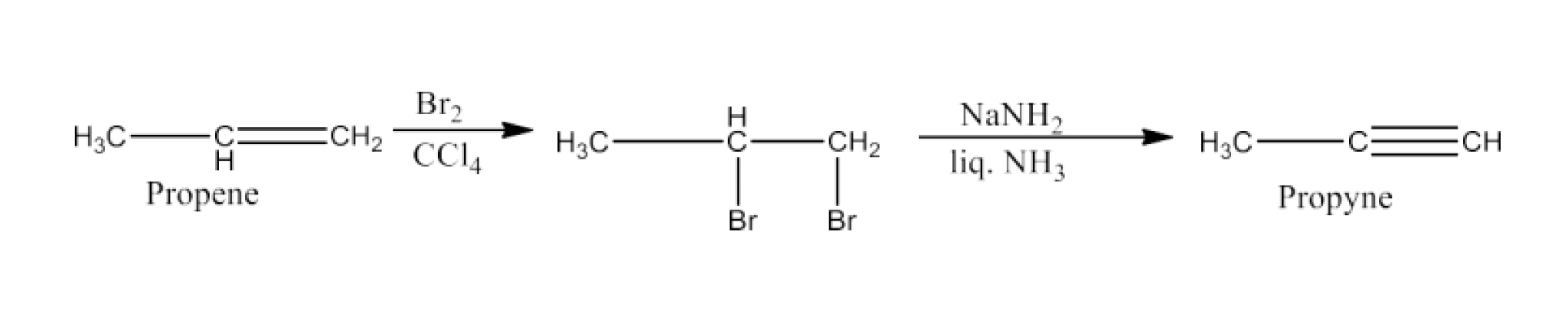
(v) Propene to propyne
Ans: Propene will react with bromine in the presence of carbon tetrachloride to give 1, 2-Dibromopropane. Now, 1, 2-Dibromopropane will react with NaNH2and liquid ammonia to give propyne. The reaction is given below:
(vi)Ethanol to ethyl Fluoride
Ans: Ethanol will react with SOCl2and pyridine to form ethyl chloride. Now, ethyl chloride will react with Hg F2 2to form Ethyl fluoride. The reaction is given below:
$CH_3CH_2OH \overset{SOCI_2, Pyridine} \rightarrow CH_3CH_2-CI \overset{Hg_2F_2} \rightarrow CH_3CH_2-F$
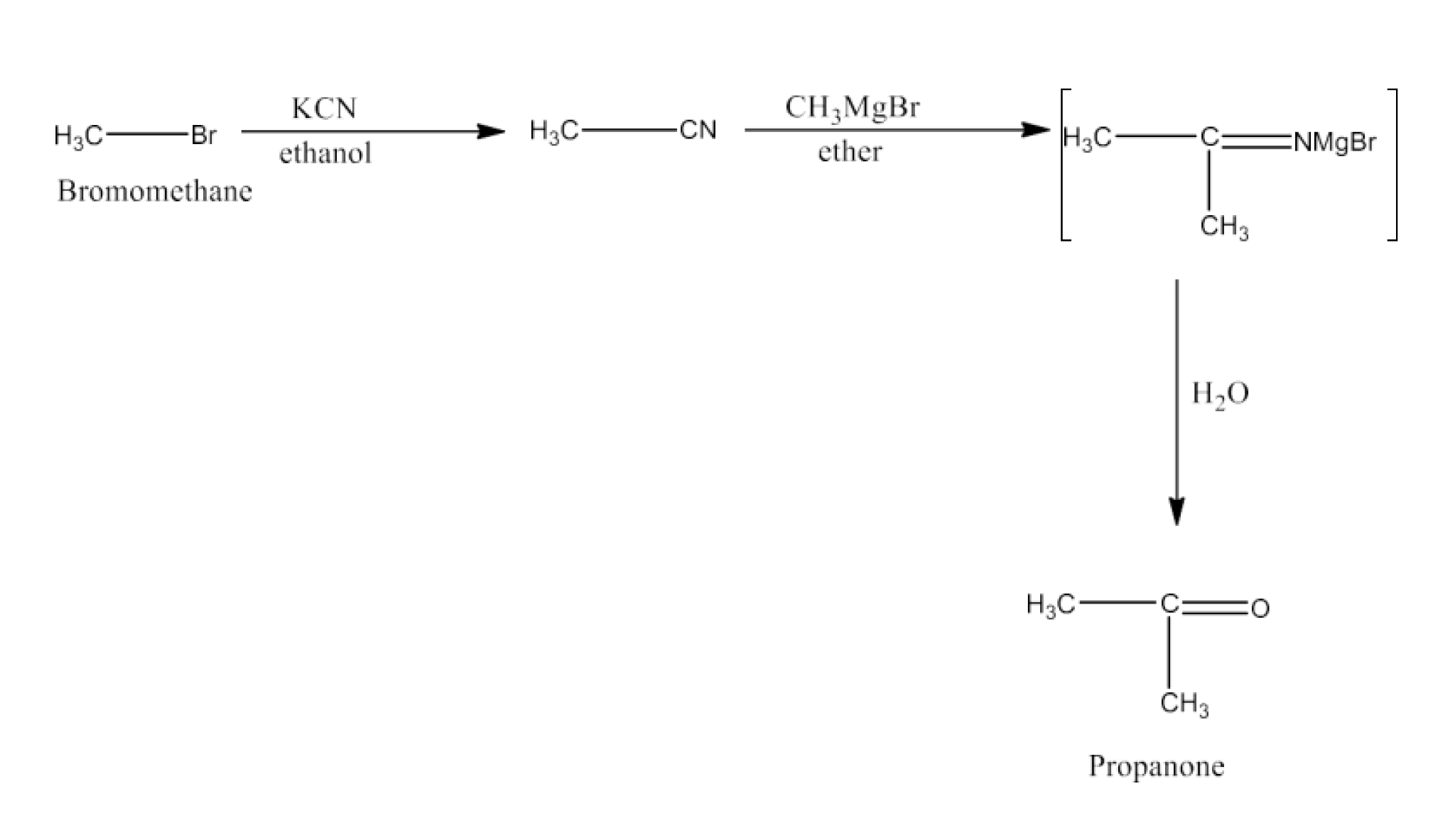
(vii) Bromomethane to Propanone
Ans: Bromomethane will react with KCN in the presence of ethanol to form acetonitrile. Acetonitrile will react with CH MgBr 3in the presence of ether and then with water to give Propanone. The reaction is given below:
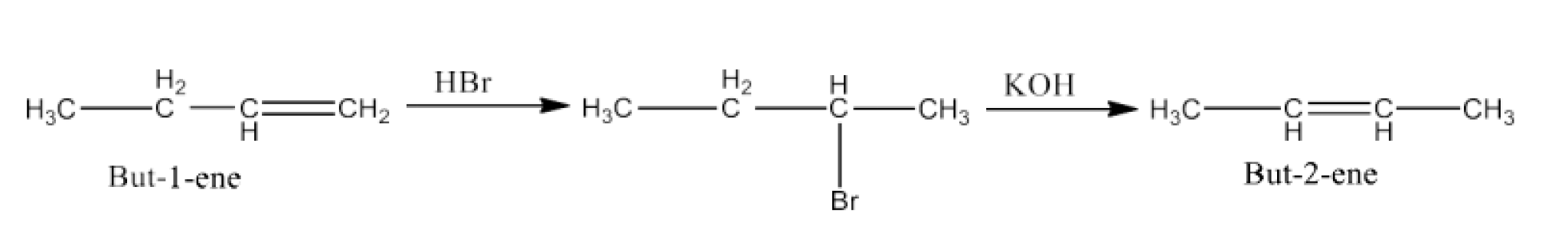
(viii) But-1-ene to but-2-ene
Ans: But-1-ene will react with Hydrogen bromide to form 2-Bromobutane and this will react with alcoholic KOH to form But-2-ene. The reaction is given below:
(ix)1-Chlorobutane to n-octane
Ans: 1-Chlorobutane will react with sodium and dry ether to form n-octane. The reaction is given below:
$2CH_3CH_2CH_2CH_2Cl + 2Na \overset{Dry\, \, etere}\rightarrow CH_3(CH_2)_6CH_3 + 2Nacl$
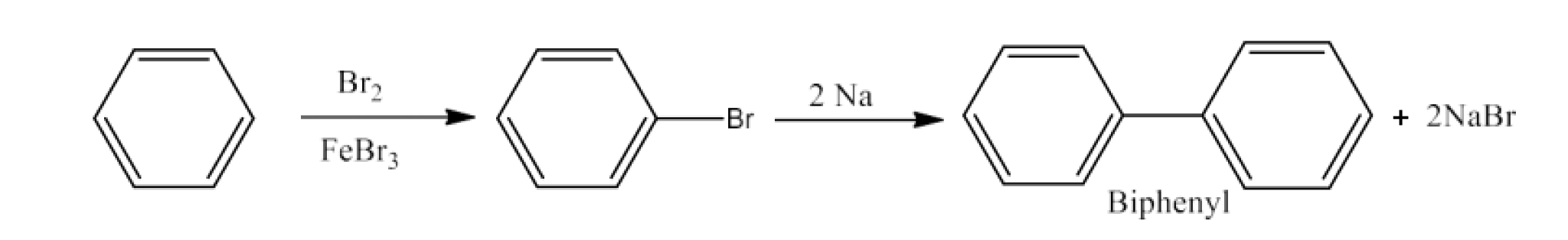
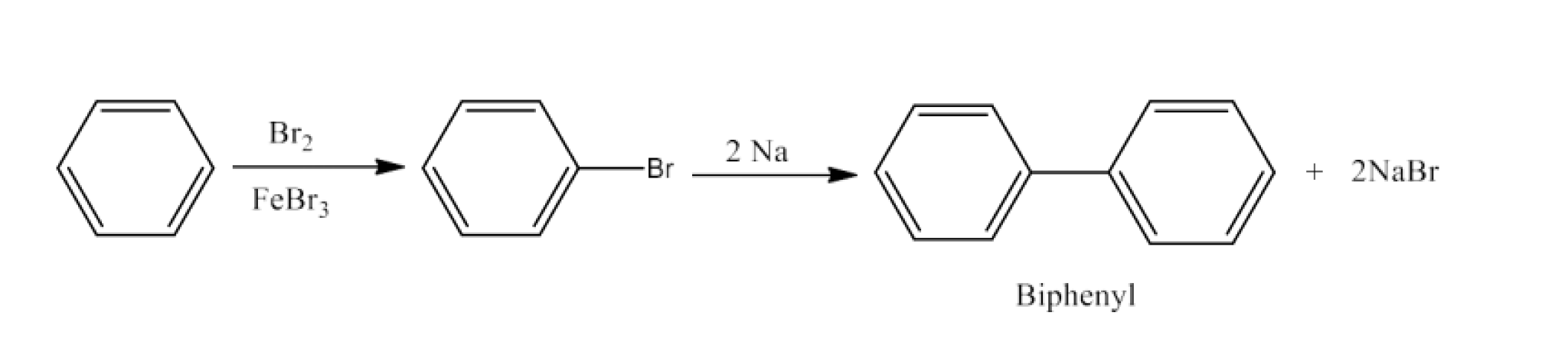
(x) Benzene to biphenyl
Ans: Benzene will react with Bromine in the presence of FeBr3to form Bromobenzene. Bromobenzene will react with sodium to form Biphenyl. The reaction is given below:
12. Explain why
(i)The dipole moment of chlorobenzene is lower than that of cyclohexyl chloride?
Ans: S-character increases electronegative properties of chlorobenzene 2
sp -hybrid
carbon. Because of this, the chlorobenzene C atom is less likely than the cyclohexyl chloride C atom to release electrons to Cl. C-Cllink in chlorobenzene is hence less polar than cyclohexyl chloride. In addition, the C-Clbond in chlorobenzene has a double bond character due to the delocalization of Cl's lone electron pairs across the benzene ring as opposed to cyclohexyl chloride, which has a pure single bond. Or to put it another way, the chlorobenzene C-Clbond is shorter than that of the cyclohexyl chloride. Considering that the dipole moment is a function of charge and distance. C-Cldistance is shorter in chlorobenzene, therefore it has a smaller dipole moment than cyclohexyl chloride.
(ii) Alkyl halides, though polar, are immiscible with water?
Ans: Dipole-dipole attraction holds alkyl halides together.
H-bonds hold the molecules of HO2 together. Alkyl halides are not soluble in water because the new forces of attraction between water and alkyl halide molecules are lower than the existing forces of attraction between alkyl halide - alkyl halide molecules and water-water molecules.
In addition, alkyl halides are unable to establish hydrogen bonds with water or to disrupt the H-bonding network that exists in water.
(iii)Grignard reagents should be prepared under anhydrous conditions?
Ans: The reactivity of the Grignard reagent is very high. When they come in contact with water they readily react and form alkanes. The general reaction is given below:
R-Mg-X + H-OH→ R-H + Mg(OH)X
Therefore, the Grignard reagent should be made in anhydrous condition.
13. Give the uses of Freon-12, DDT, carbon tetrachloride and iodoform.
Ans:
Iodoform:
Early on it was considered to be an antiseptic, however the characteristics are attributable to the free iodine that is released, not the substance itself. It has been superseded by other iodine-containing formulations due to its offensive odour.
Carbon Tetrachloride:
For oil, fats, and resins in the industrial sector, as well as in dry cleaning. In addition, CCl4vapours are extremely inflammable, according to the manufacturer. As a result, CCl4is sold as pyrene, a fire extinguishing agent.
Used in the production of aerosol can refrigerants and propellants.
Freons:
Industry uses Freon-12 (CCl F2 2), which is the most prevalent form of refrigerant. Refrigerant or air-conditioning components, aerosol propellants
DDT:
Its efficacy against mosquitoes that carry malaria and other insects that harm crops led to a dramatic increase in its usage worldwide following World War II. DDT, on the other hand, has been widely used since the 1940s. Toxic for fishes, DDT acquired tolerance in many insect species. When it comes to animals, DDT is not readily metabolised, but instead accumulates and is retained in fatty tissues. As long as the animals continue to eat DDT at the same rate, it builds up in their bodies.
14. Write the structure of the major organic product in each of the following reactions:
(i)$\mathbf{CH_3CH_2CH_2Cl +NaI \overset{Acetone, Heat}\rightarrow} $
Ans: The Chlorine atom from the alkyl halide by iodine. The major product of the reaction is 1-Iodopropane. The reaction is given below:
$CH_3CH_2CH_2Cl + NaI \overset{Acetone, Heat}\rightarrow CH_3CH_2CH_2 I + NaCl$
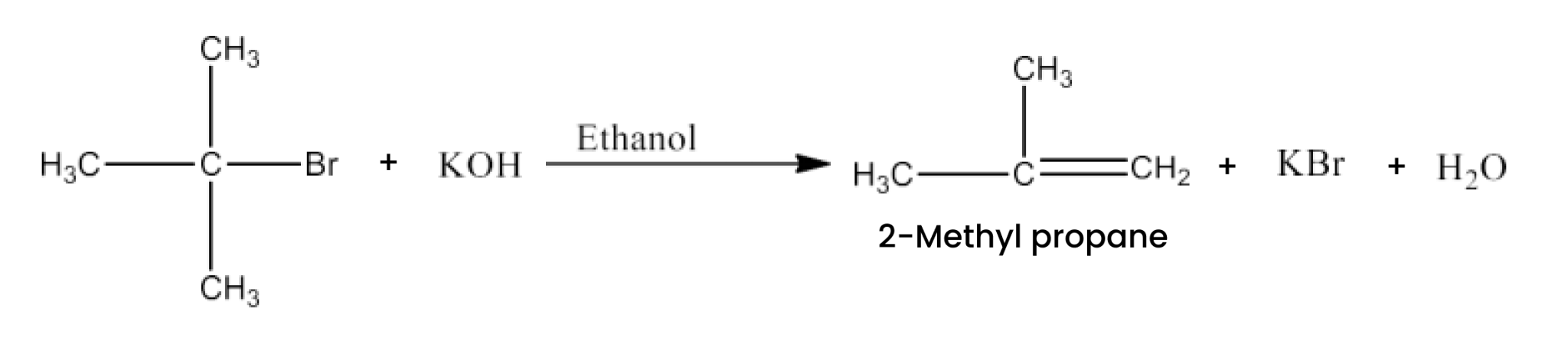
(ii)$\mathbf{(CH_3)_3CBr + KOH \overset{Ethanol}\rightarrow }$
Ans: There will be Dehydrohalogenation and the major product of the reaction is 2- methylpropene. The reaction is given below:
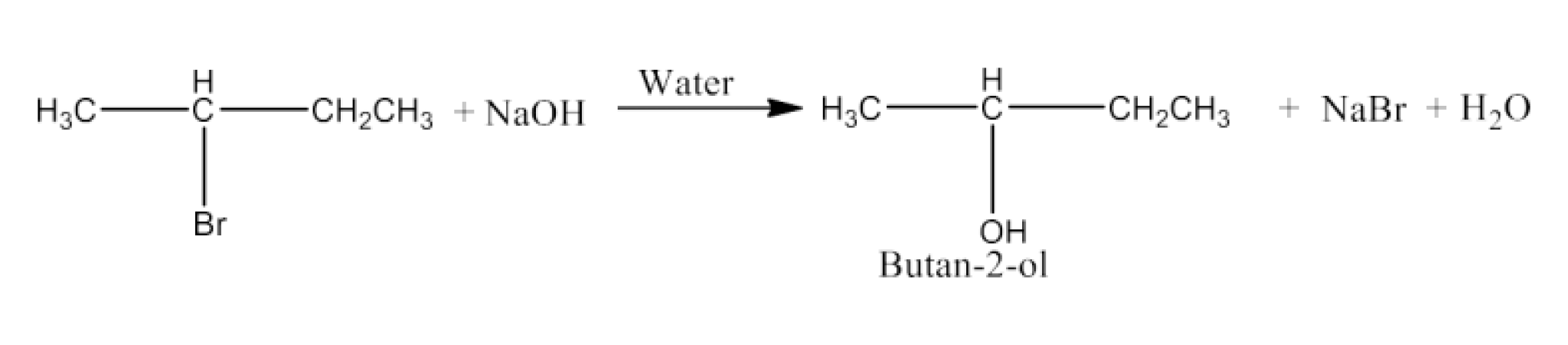
(iii)$\mathbf{C_3CH(Br)CH_2CH_3 + NaOH \overset{water}\rightarrow }$
Ans: The bromine atom will be replaced with the hydroxyl ion. The major product will be Butan-2-ol. The reaction is given below:
(iv)$\mathbf{CH_3CH_2Br +KCN \overset{aq.ethanol } \rightarrow} $
Ans: The bromide ion will be replaced with the cyanide ion. The major product will be propanenitrile. The reaction is given below:
$CH_3CH_2Br +KCN \overset{aq.ethanol } \rightarrow CH_3CH_2CN + KBr$
(v)$\mathbf{C_6H_5ONa + C_2H_5Cl \rightarrow} $
Ans: The major product in the above reaction will be Phenetole. The reaction is given below:
$C_6H_5ONa + C_2H_5Cl \rightarrow C_6H_5-O-C_2H_5+ NaCl $
(vi)CH3CH2CH2OH + SOCl2 →
Ans: The hydroxyl ion in the alkyl halide will be replaced with chloride ion. The major product of the reaction will be 1-Chloropropane. The reaction is given below:
CH3CH 2CH2OH + SOCl2 → CH3 CH2CH2Cl + SO2 + HCl
(vii)$\mathbf{CH_3CH_2CH=CH_2+HBr \overset{peroxide}\rightarrow} $
Ans: Since peroxide is used in the reaction, there will be Anti-Markovnikov's addition. The major product in the reaction will be 1-Bromobutane. The reaction is given below:
$CH_3CH_2CH=CH_2+HBr \overset{peroxide}\rightarrow CH_3CH_2CH_2CH_2Br$
(viii)CH3CH=C(CH3)2 + HBr →
Ans: In this reaction there will be Markovnikoff’s addition. The major product of the reaction will be 2-Bromo-2-methylbutane. The reaction is given below:
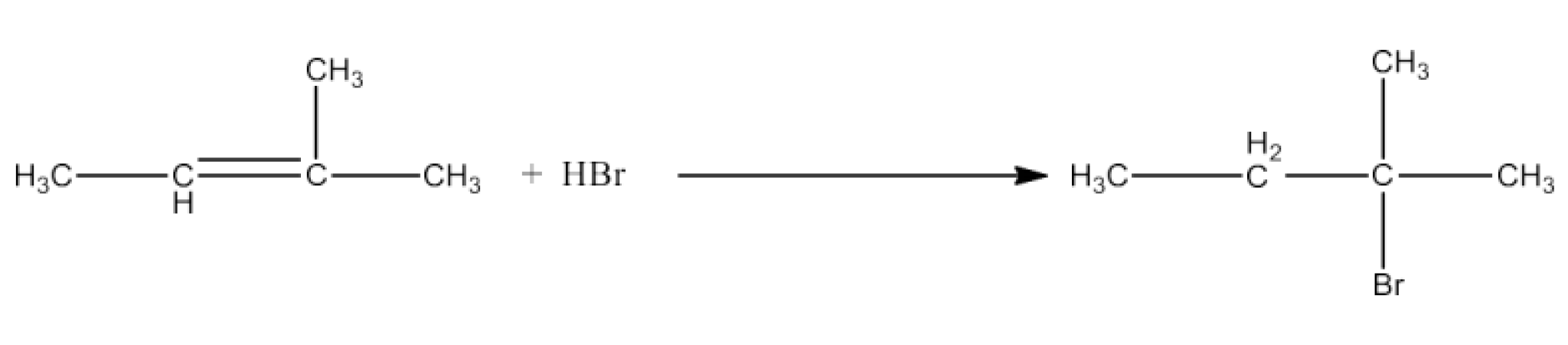
15. Write the mechanism of the following reaction:
$\mathbf{n-BuBr + KCN \overset{EtoH-H_2O}\rightarrow n BuCN}$
Ans: There are two contributing resonance hybrid structures of KCN due the cyanide ion present. It is given below:
$K^{+}[-: C=N:\leftrightarrow :C=N:-]$
So, as we can see that the - CNion is an ambident nucleophile. Therefore, it can attack from either C atom or from N atom towards n-BuBr. The strength of C-C bond is stronger than C-N bond, therefore, the attack will take place from C atom towards n Butyl bromide to form n-butyl cyanide. The reaction is given below:
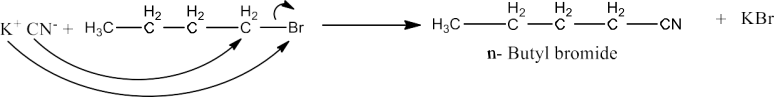
16. Arrange the compounds of each set in order of reactivity towards SN2 displacement:
Ans: For the SN2 reaction the order of reactivity is 1 2 3 > >, so we can solve the question according to the order of the reactivity.
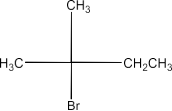
(i)2-Bromo-2-Methylbutane, 1-Bromopentane, 2-Bromopentane.
First let us draw all the structures of the compound.
2-Bromo-2-Methyl butane:
1-Bromopentane:
CH CH CH CH CH -Br 3 2 2 2 2
2-Bromopentane:
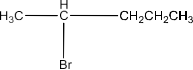
The order will be:
1-Bromopentane > 2-Bromopentane > 2-Bromo-2-methyl butane.
(ii) 1-Bromo-3-methylbutane,2-Bromo-2-methylbutane,3-Bromo-2- methylbutane
Ans: The structures of the compounds are given below:
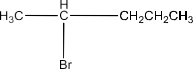
1-Bromo-3-methylbutane:
2-Bromo-2-methylbutane:
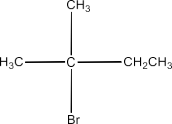
3-Bromo-2-methylbutane:
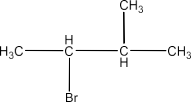
The order of the reactivity will be:
1-Bromo-3-methylbutane > 3-Bromo-2-methylbutane > 2-Bromo-2-methyl butane.
(iii)1-Bromobutane, 1-Bromo-2,2-dimethypropane, 1-Bromo-2-methylbutane, 1- Bromo-3-methyl butane.
Ans: The structure of the compounds are given below:
1-Bromobutane:
CH3 CH2 CH2 CH2 Br
1-Bromo-2, 2-dimethylpropane:
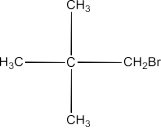
1-Bromo-2-methylbutane:
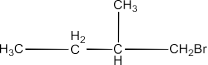
1-Bromo-3-methylbutane:
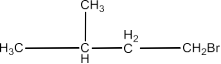
The order of reactivity will be:
1-Bromobutane > 1-Bromo-3-methylbutane > 1-Bromo-2-methylbutane > 1-Bromo-2, 2-dimethylpropane.
This is decided by the fact that steric hindrance should be less for S 2Nfor the reactivity.
17. Out of C6 H5CH2Cl and C6H5CHClC6H5 which is more easily hydrolyzed by aqueous KOH.
Ans: C6H5CHCl2 is a primary aryl halide but C6H5CHClC6H5 is a secondary aryl halide. If reaction with aqueous KOH is a SN1 reaction then it is based on the stability of the carbocation. The formation of carbocation is given below:
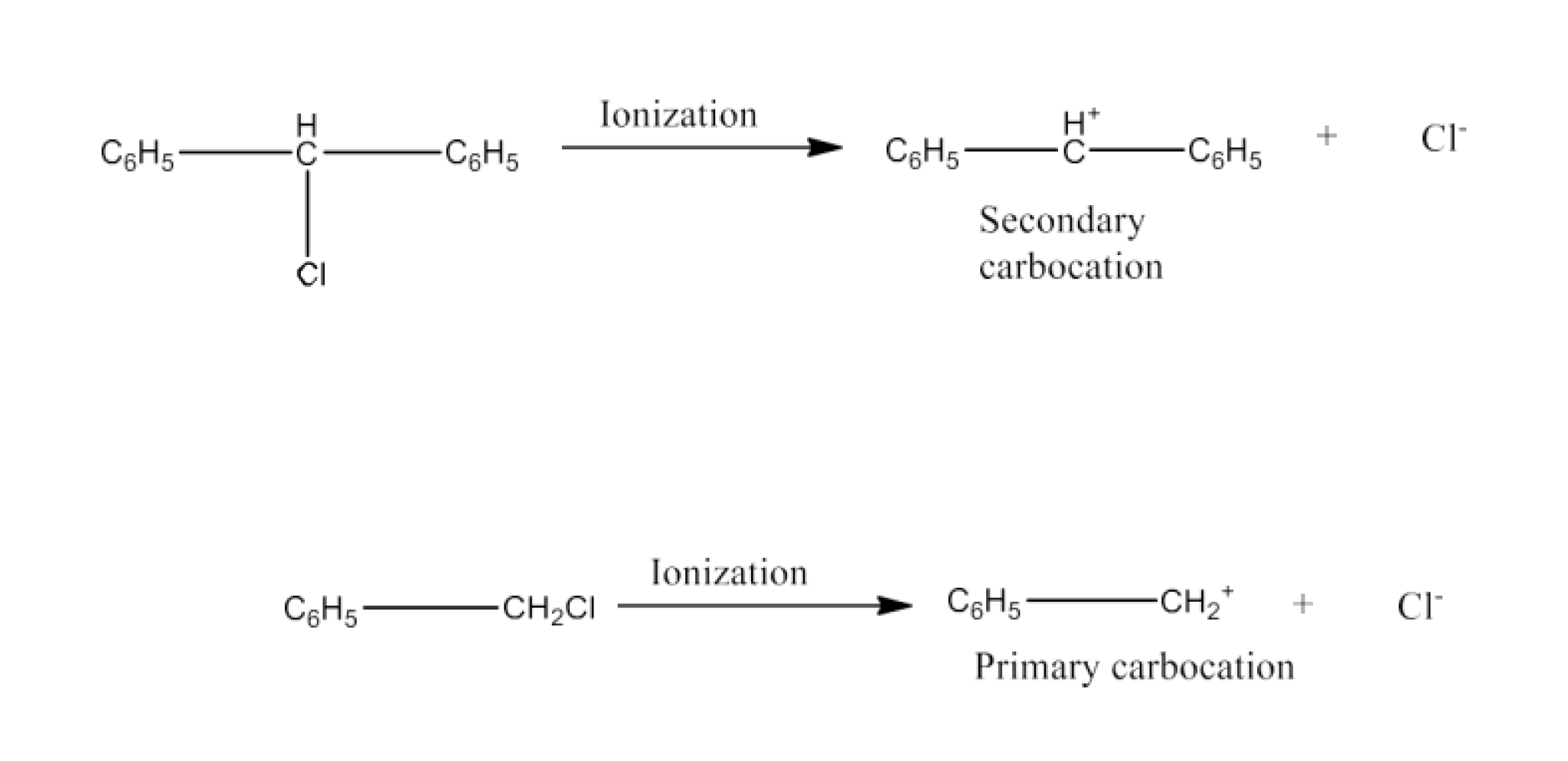
Secondary carbocation is more stable than primary carbocation so, the C6H5CHClC6H5 will react faster.
But if the reaction is SN2 then C6H5CHCl2 will react faster because the reactivity is of opposite order.
18. p-Dichlorobenzene has higher m.p. and lower solubility than those of o-and m-isomers. Discuss.
Ans: There are greater molecular forces of attraction between p-isomers and o-isomers due to it being more symmetrical and fitting into the crystal lattice more tightly. Due to the fact that the crystal lattice breaks during melting or dissolution, a greater amount of energy is required to melt or dissolve the p-isomer than the o- and m-isomers. This means that p-isomers have higher melting points and lower solubilities than their equivalent o- and m-isomers.
19. How the following conversions can be carried out:
(i)Propene to propan-1-ol
Ans: Propene will react with hydrogen bromide in the presence of peroxide to give 1- Bromopropane. Now, this 1-Bromopropane will react with aqueous KOH to form Propan-1-ol. The reaction is given below:
$CH_3CH=CH_2\overset{HBr/ peroxide}\rightarrow CH_3CH_2CH_2Br\overset{Aq. KOH}\rightarrow CH_3CH_2CH_2OH$
(ii) Ethanol to but-2yne
Ans: Ethanol will react with iodine and phosphorus to form Iodoethane then it will convert to ethene. Now ethene will react with bromine in the presence of carbon tetrachloride to form1, 2-Dibromoethane, this on dehydrohalogenation and reaction
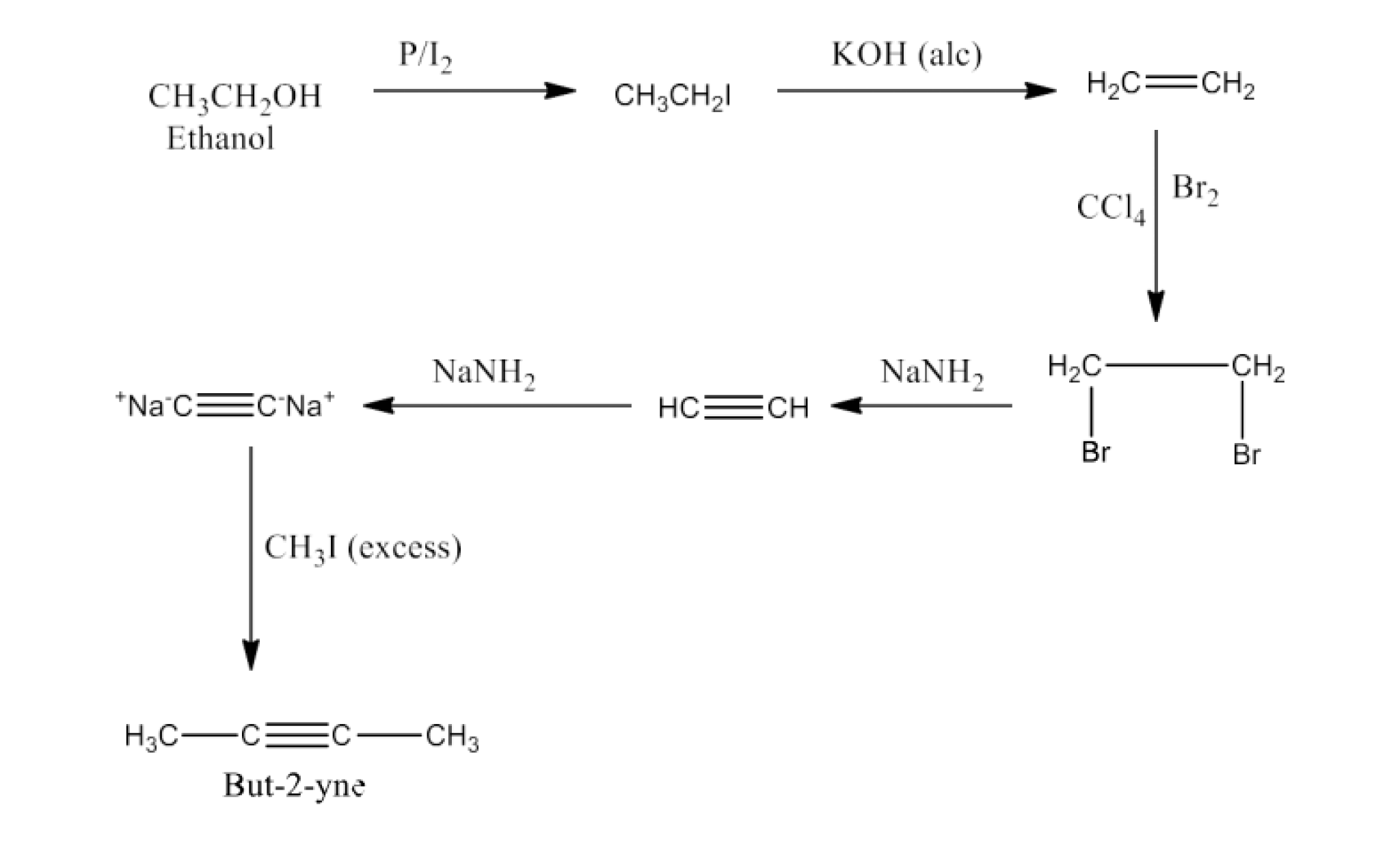
with NaNH2will form disodium acetylide. Later this reaction with methyl iodide will give But-2-yne. The reaction is given below:
(iii)1-Bromopropane to 2-bromopropane
Ans: 1-Bromopropane will react with alcoholic KOH to form propene. Propene will react with HBr to form 2-Bromopropane. The reaction is given below:
$CH_3CH_2CH_2Br\overset{Alc.KOH}\rightarrow CH_3CH=CH_2 \overset{HBr}\rightarrow CH_3-CH(Br)-CH_3$
(iv) Toluene to Benzyl alcohol.
Ans: Toluene will react with chlorine in the presence of light to give benzyl chloride and this benzyl chloride will react with aqueous KOH to give benzyl alcohol. The reaction is given below:
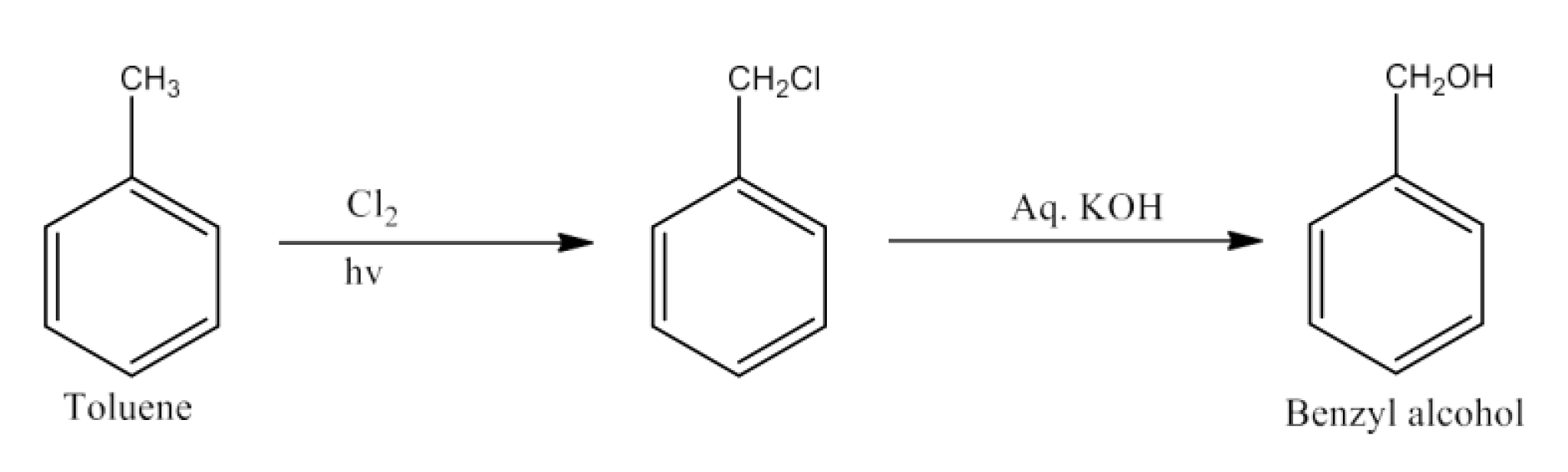
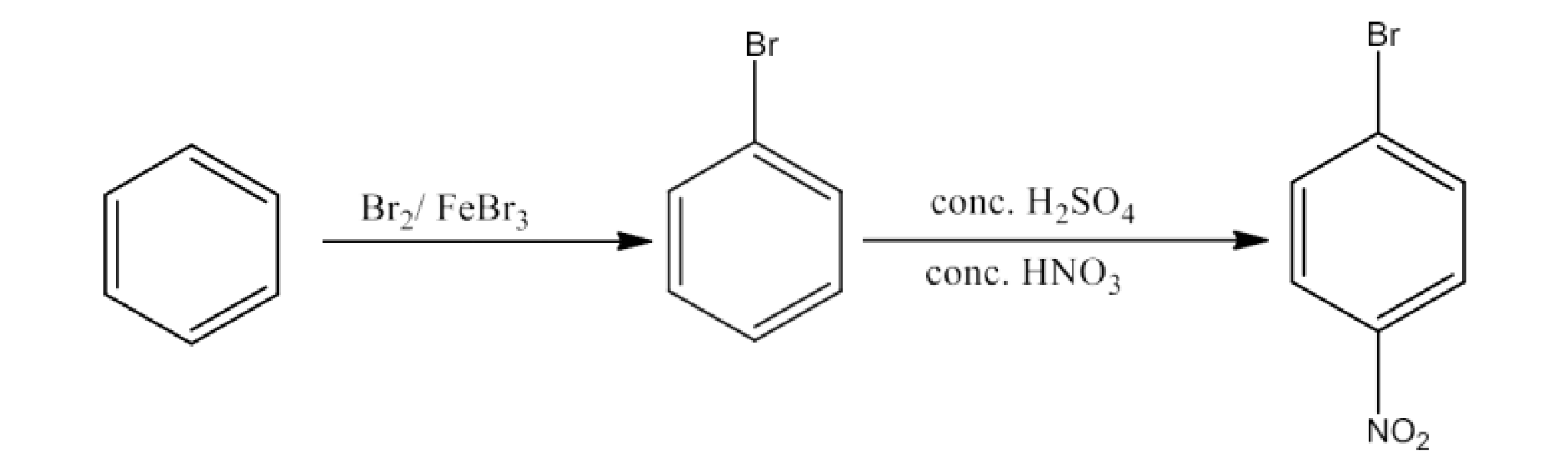
(v) Benzene to 4-bromonitrobenzene
Ans: Benzene will react with Bromine in the presence of FeBr3to form Bromobenzene. Bromobenzen will react with concentrated nitric acid and concentrated sulfuric acid to form 4-Bromonitrobenzene. The reaction is given below:
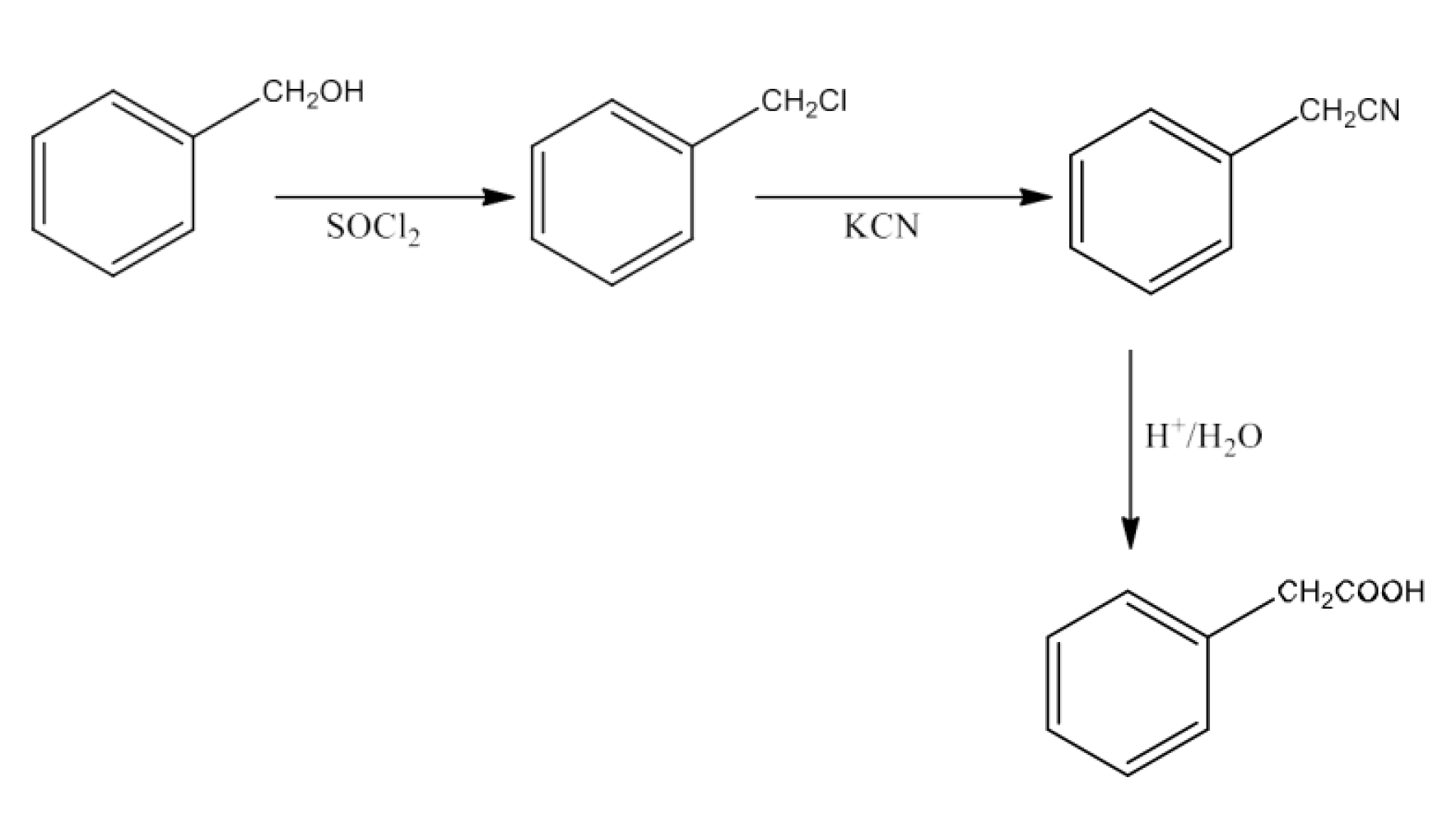
(vi) Benzyl alcohol to 2-phenylethanoic acid
Ans: Benzyl alcohol will react with SOCl2to form Benzyl chloride. Benzyl chloride will react with KCN to form Benzyl cyanide. Now, benzyl cyanide on hydrolysis will give 2-Phenylethanoic acid. The reaction is given below:
(vii) Ethanol to Propanenitrile
Ans: Ethanol will react with iodine in the presence of phosphorus to form Iodoethane. Iodoethane will react with KCN in the presence of aqueous ethanol to give Propanenitrile. The reaction is given below:
$CH_3CH_2OH \overset{p/I_2}\rightarrow CH_3CH_2I \overset{KCN/aq.Ethanol}\rightarrow CH_3CH_2CN$
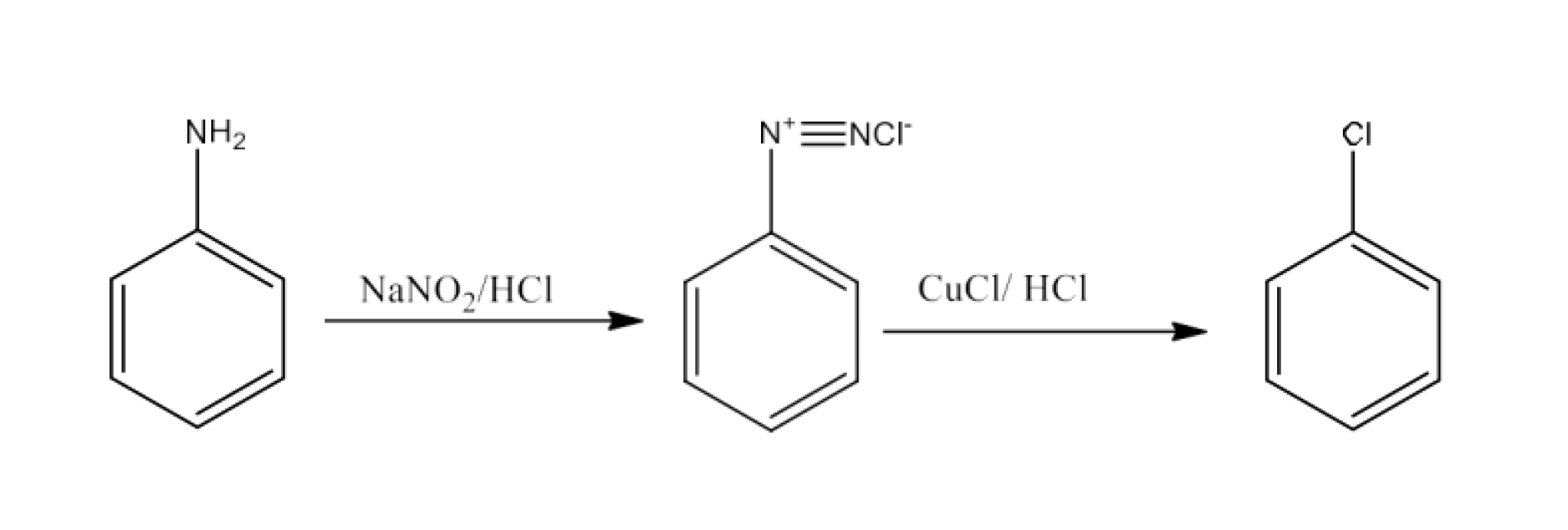
(viii) Aniline to Chlorobenzene
Ans: Aniline will undergo diazotization to form Benzene diazonium chloride. The Benzene diazonium chloride will react with copper chloride in the presence of hydrochloric acid to give Chlorobenzene. The reaction is given below:
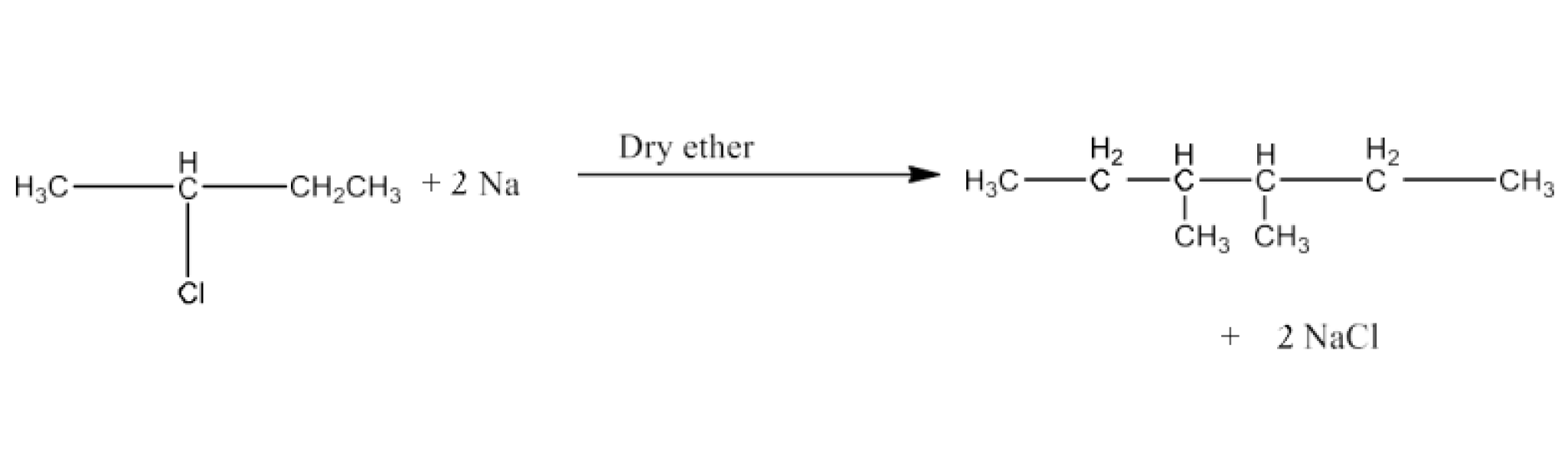
(ix) 2-Chlorobutane to 3, 4-Dimethylhexane
Ans: 2-Chlorobutane will react with sodium in the presence of dry ether to form 3, 4- Dimethylhexane. The reaction is given below:
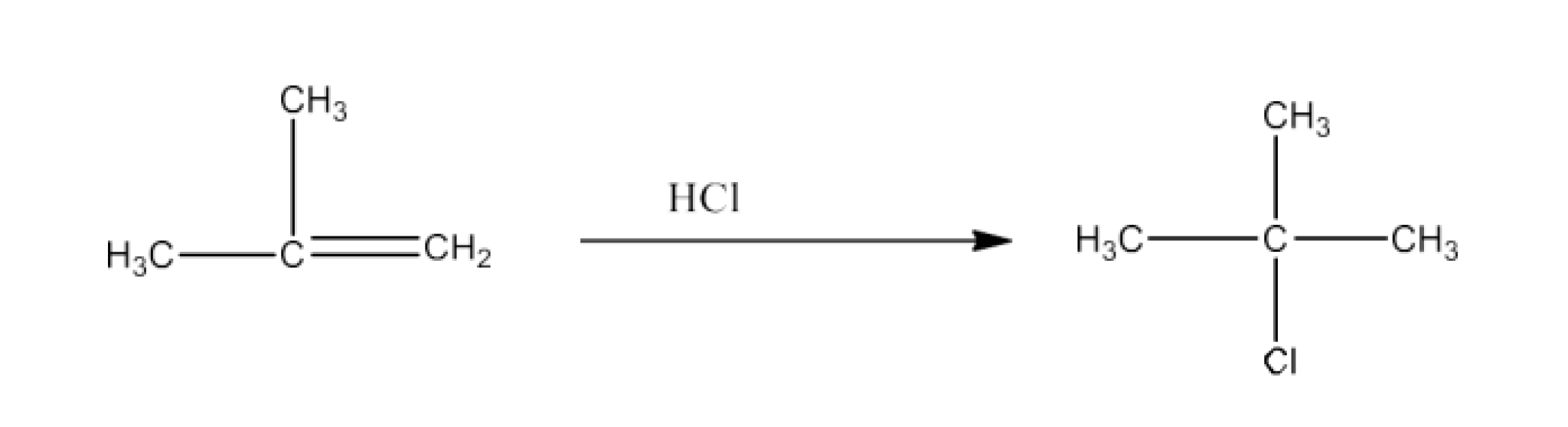
(x) 2-Methyl-1-propene to 2-Chloro-2-methylpropane
Ans: 2-Methyl-1-propene will react with Hydrogen chloride to give 2-Chloro-2- methylpropane. The reaction is given below:
(xi) Ethyl chloride to propanoic acid
Ans: Ethyl chloride will react with KCN to give propanenitrile. Propanenitrile on hydrolysis will give propanoic acid. The reaction is given below:
$CH_3CH_2Cl \overset{KCN}\rightarrow CH_3CH_2CN \overset{H^{+}/H_2O}\rightarrow CH_3CH_2COOH$
(xii) But-1-ene to n-butyl iodide
Ans: But-1-ene will react with HBr in the presence of peroxide to form 1- Bromobutane. 1-Bromobutane will react with NaI in the presence of Acetone to give n-butyl iodide. The reaction is given below:
$CH_3CH_2CH=CH_2 \xrightarrow[peroxide]{HBr}CH_3CH_2CH_2CH_2Br \xrightarrow[Acetone]{Nal} CH_3CH_2CH_2CH_2I$
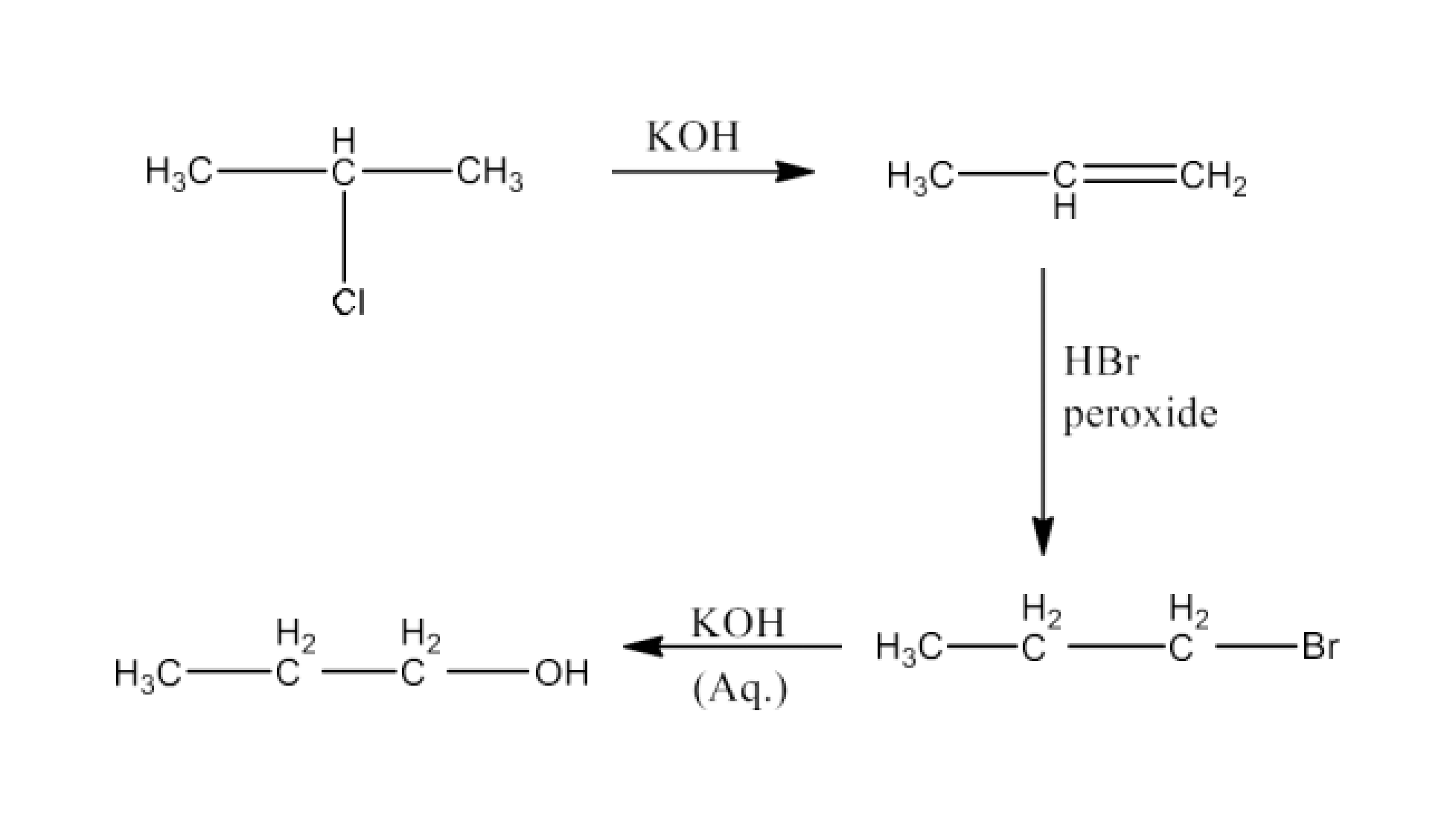
(xiii) 2-Chloropropane to 1-propanol
Ans: 2-Chloropropane will undergo dehydrohalogenation to give propene. Propene will react with HBr in the presence of peroxide to give 1-bromopropane. 1- Bromopropane will react with KOH to form 1-Propanol. The reaction is given below:
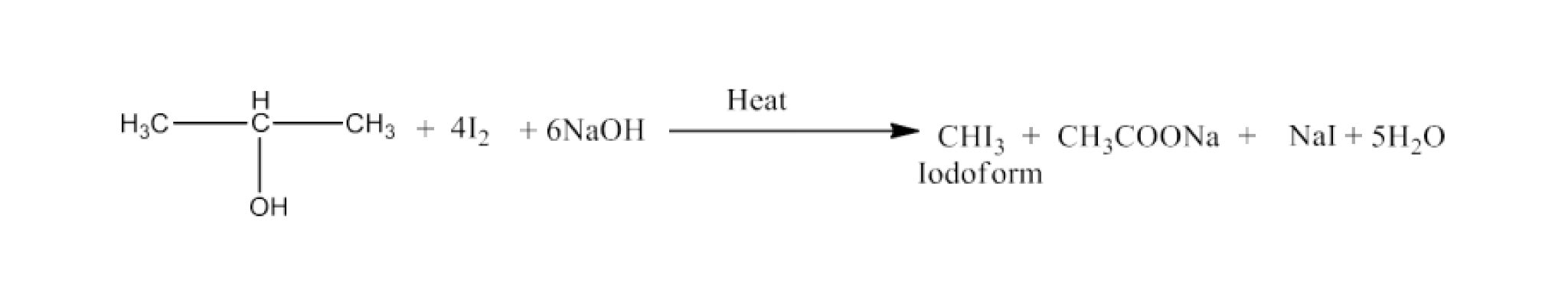
(xiv) Isopropyl alcohol to Iodoform
Ans: Isopropyl alcohol will react with iodine and sodium hydroxide to give Iodoform. The reaction is given below:
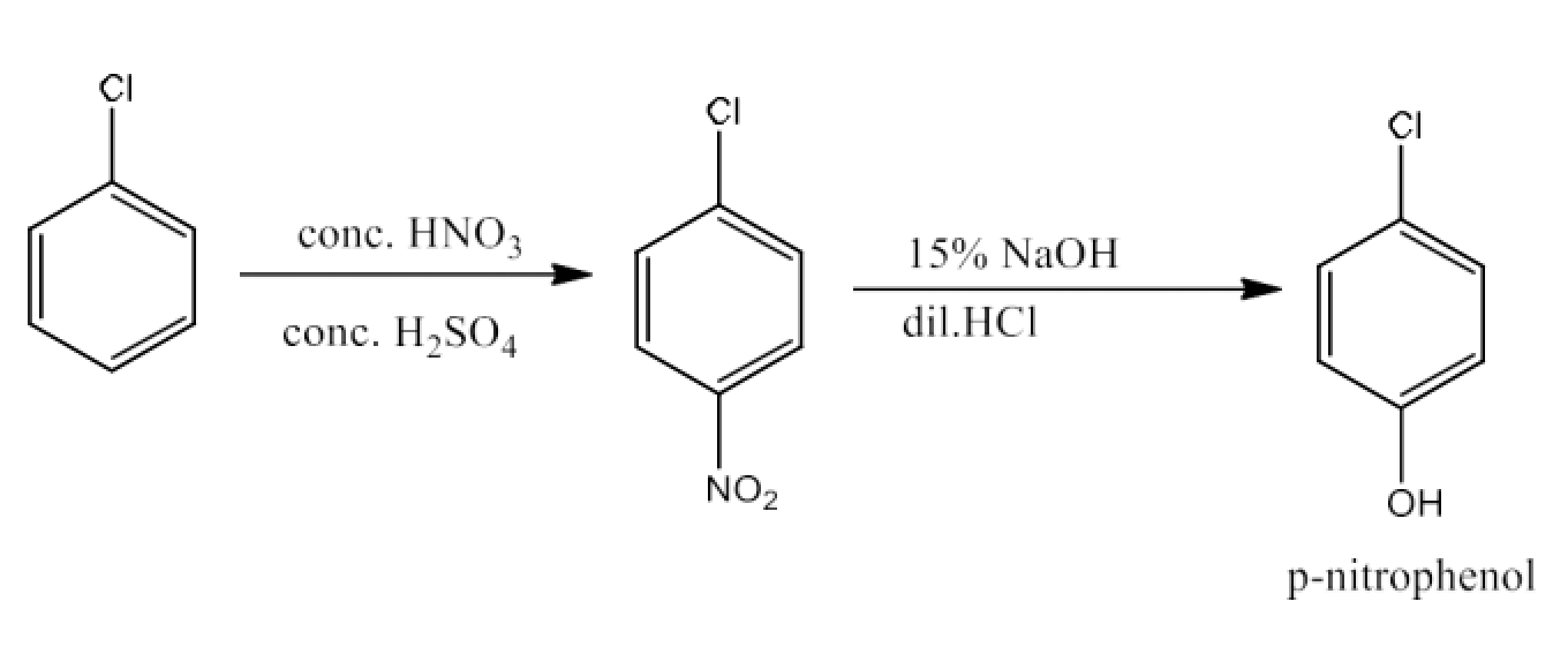
(xv) Chlorobenzene to p-nitrophenol
Ans: Chlorobenzene will react with concentrated nitric acid and concentrated sulfuric to give p-Chloronitrobenzene. p-Chloronitrobenzene will react with 15% sodium hydroxide and hydrochloric acid to give p-nitrophenol. The reaction is given below:
(xvi)2-Bromopropane to 1-bromopropane
Ans: 2-Bromopropane will react with alcoholic KOH to form Propene. Now, propene will react with HBr in the presence of peroxide to form 1-Bromopropane. The reaction is given below:
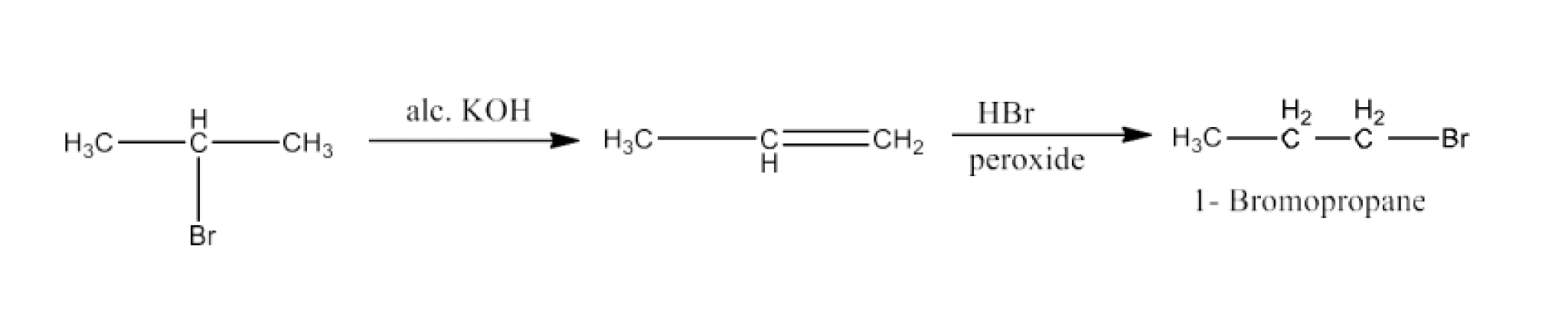
(xvii) Chloroethane to butane
Ans: Chloroethane will react with sodium in the presence of butane. The reaction is given below:
$2CH_3CH_2-Cl + 2Na \overset{ Dryethere}\rightarrow CH_3CH_2CH_2CH_3 + 2NaCl$
(xviii) Benzene to diphenyl
Ans: Benzene will react with Bromine in the presence of FeBr3to form Bromobenzene. Bromobenzene will react with sodium to form Biphenyl. The reaction is given below:
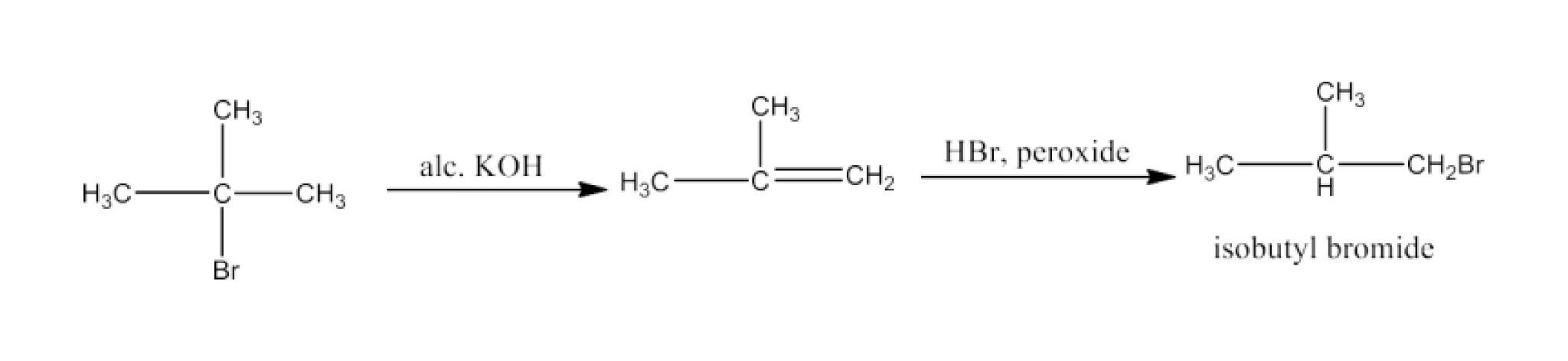
(xix)Tert-Butyl bromide to isobutyl bromide
Ans: Tert-butyl bromide will react with alcoholic KOH to form 2-Methyl-1-propene. 2-Methyl-1-propene will react with HBr in the presence of peroxide to form isobutyl bromide. The reaction is given below:
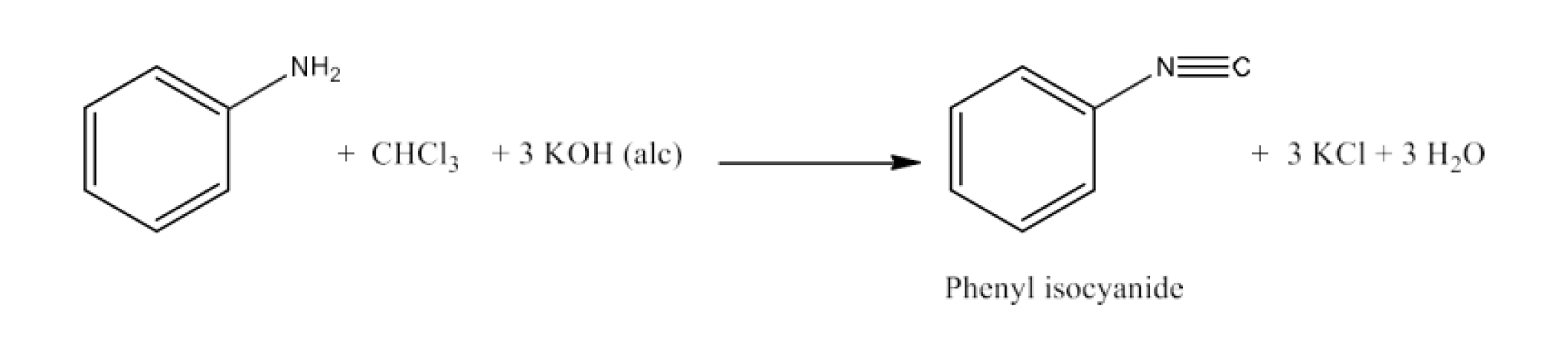
(xx) Aniline to phenylisocyanide
Ans: Aniline will react with chloroform and alcoholic KOH to form Phenyl isocyanide. The reaction is given below:
20. The treatment of alkyl chlorides with aqueous KOH leads to the formation of alcohols but in the presence of alcoholic KOH, alkenes are major products. Explain.
Ans: Because of its high nucleophilicity, KOH is nearly fully deionized in water to generate - OHions, which then undergo substitution reactions with alkyl halides to form alcohols.
OH- ions are strongly solvated in the aqueous solution (hydrated). A result of this process is that alkyl chloride fails to extract a hydrogen from its p-carbon in order to create alkenes. While an alcohol-based solution of KOH contains an ion known as the alkoxide (- RO), this strong base preferentially removes HCI from an alkyl chloride to produce alkenes.
21. Primary alkyl halide C4H9Br(a) reacted with alcoholic KOH to give compound (b) Compound (b) is reacted, with HBr to give (c) which is an isomer of (a). When (a) is reacted with sodium metal it give compound (d), C8 H18which is different from the compound formed when n-butyl bromide is reacted with sodium. Give the structural formula of (a) and write the equations for all the reactions.
Ans: C4H9Br is the chemical formula for two main primary alkyl halides. These are given below:
n-butyl bromide: CH3CH2CH2CH2Br
and Isobutyl bromide:
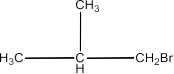
As compound (a) when reacted with Na metal generated a compound (d) with a molecular formula C H8 18which was different from the molecule obtained when n butyl bromide was reacted with Na metal, hence (a) must be isobutyl bromide and compound (d) must be n-butyl bromide.
The reaction will be:
$2CH_3CH_2CH_2CH_2Br + 2Na \overset{Wurtz reaction}\rightarrow CH_3(CH_2)_6CH_3$
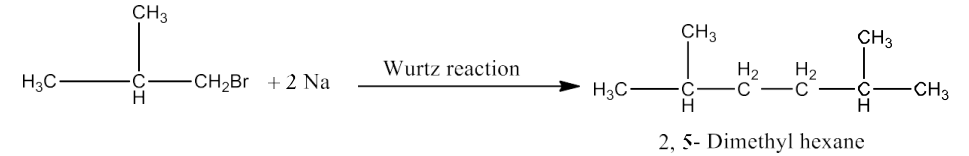
Isobutyl bromide is compound (a). Because of this, 2-methyl-1-propane must be the chemical (b) that it yields after being treated with alcohol KOH. The reaction is given below:
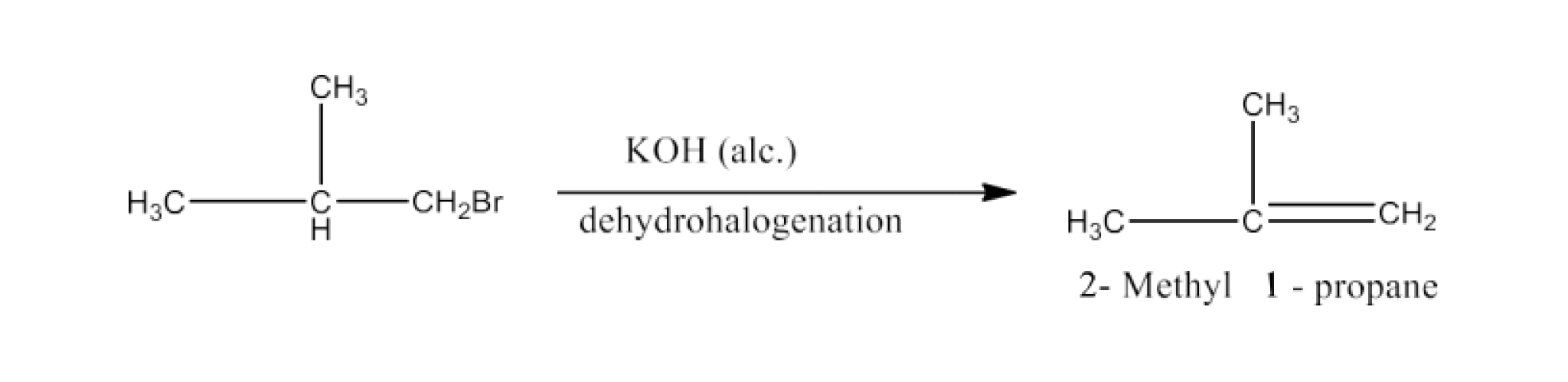
On treatment with HBr, compound (b) transforms into compound (c) in line with the Markownikoff rule To summarise: Compound (c), also known as tert- butyl bromide, is an isomer of compound (a), also known as isobutyl bromide.
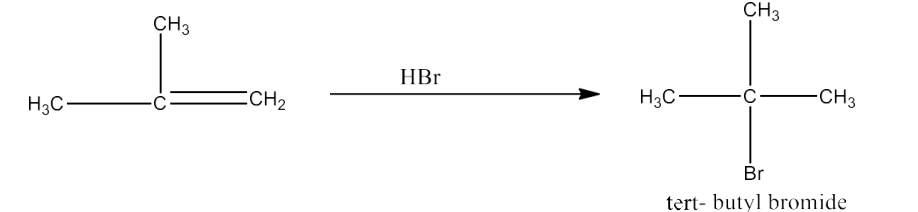
Therefore, (a) is isobutyl bromide, (b) is 2-Methyl-1-propane, (c) is tert-butyl bromide, and (d) is 2, 5-Dimethylhexane.
22. What happens when.
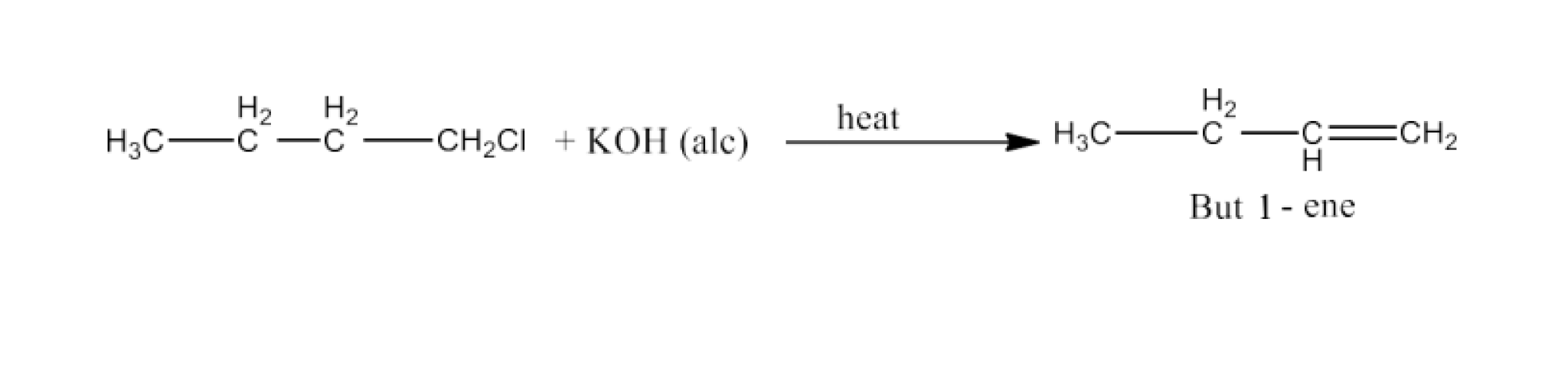
(i)n-butyl chloride is treated with alcoholic KOH.
Ans: When n-butyl chloride is treated with alcoholic KOH, then there will be dehydrohalogenation and the product will be But-1-ene. The reaction is given below:
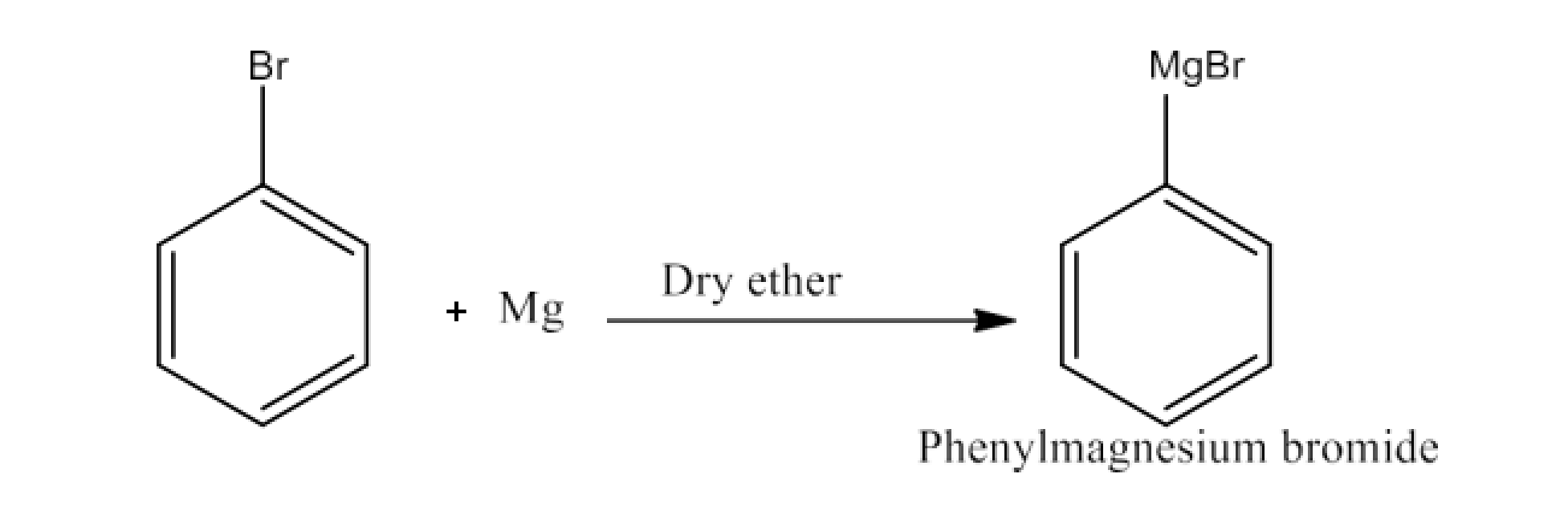
(ii) Bromobenzene is treated with Mg in the presence of dry ether.
Ans: When bromobenzene is treated with Mg in the presence of dry ether there will be the formation of Phenylmagnesium bromide. The reaction is given below:
(iii)Chlorobenzene is subjected to hydrolysis.
Ans: When chlorobenzene is hydrolyzed then there will be no reaction.
(iv)Ethyl chloride is treated with aqueous KOH
Ans: When ethyl chloride is treated with aqueous KOH then the product will be Ethyl alcohol. The reaction is given below:
$CH_3CH_2Cl + KOH (aq) \overset{Hydrolysis}\rightarrow CH_3CH_3OH + KCl$
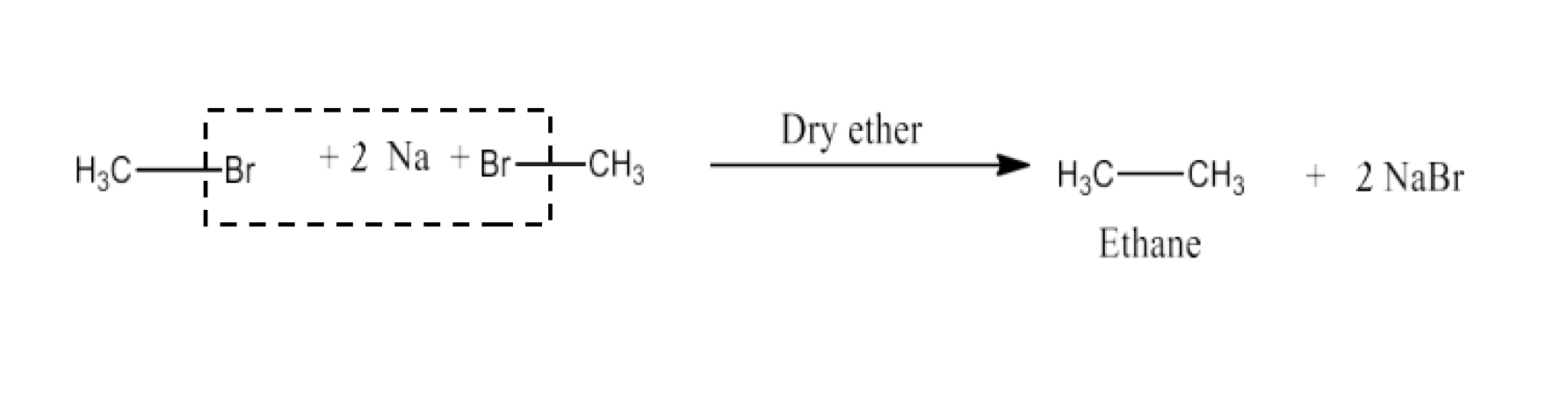
(v) Methyl bromide is treated with sodium in the presence of dry ether.
Ans: When methyl bromide is treated with sodium in the presence of dry ether then Wurtz reaction will take place and the product of the reaction will be Ethane. The reaction is given below:
(vi)Methyl chloride is treated with KCN.
Ans: When methyl chloride is treated with KCN then there will be a Nucleophilic substitution reaction and the product will be Methyl cyanide. The reaction is given below:
$CH_3-Cl + KCN \overset{ethanol}\rightarrow CH_3-C \equiv N + KCl$
Class 12 Chemistry Chapter 6 Quick Overview of Topics
Class 12 Chemistry Chapter 6 NCERT Solutions- Quick Overview of Detailed Structure of Topics and Subtopics Covered.
Topic | Subtopics |
Introduction to Haloalkanes and Haloarenes | -Definition of Haloalkanes and Haloarenes - Comparison between the two classes of compounds |
Nomenclature of Haloalkanes and Haloarenes | -IUPAC nomenclature rules for both Haloalkanes and Haloarenes and Common names and trivial names |
Nature of C-X Bond in Haloalkanes and Haloarenes | -Polar nature of C-X bond Dipole moment - Hybridization of carbon atoms in Haloalkanes and Haloarenes |
Preparation Methods | -Halogenation of alkanes from alcohols, alkenes, alkynes, carboxylic acids and their derivatives, geminal dihalides |
Class 12 NCERT Solutions Chapter 6 Important Topics
Class 12 NCERT solutions help the students to go through the Important Highlights easily. Here find the Important topics of Chapter 6 - Haloalkanes and Haloarenes to crack your exams.
Nucleophilic Substitution Reaction: Haloalkanes undergo nucleophilic substitution reactions where the halogen atom is replaced by a nucleophile. The general equation for such a reaction is:
R-X+Nu→ R-Nu + X¯
SN1 Reaction Rate Equation: For a first-order nucleophilic substitution reaction (SN1), the rate equation is given by:
Rate = k[R-X]
SN2 Reaction Rate Equation: For a second-order nucleophilic substitution reaction (SN2), the rate equation is given by
Rate = k[Nu¯|[R-X]
Hofmann Elimination (Anti-Elimination): In Hofmann elimination, the leaving group and the hydrogen atom to be removed are anti to each other. This results in the formation of the least substituted alkene. The reaction mechanism involves the E2 mechanism (bimolecular elimination).
Saytzeff Elimination (Syn-Elimination): In Saytzeff elimination, the leaving group and the hydrogen atom to be removed are syn to each other. This results in the formation of the most substituted alkene. The reaction mechanism involves the E1cb mechanism (elimination, unimolecular, conjugate base).
Benefits of Class 12 NCERT Solutions for Chemistry Chapter Chapter 6 Haloalkanes and Haloarenes
The Vedantu’s Class 12 NCERT Solutions For Chemistry Chapter Chapter 6 Haloalkanes and Haloarenes provided here in PDFs offer various benefits, including:
Detailed explanations and step-by-step solutions for all topics in Chapter 6.
Solutions curated by experienced educators to ensure accuracy and clarity.
Covers important concepts like nucleophilic substitution reactions, elimination reactions, and the stability of carbocations.
Clear and concise explanations using precise chemical terminology.
Detailed analysis of the physical and chemical properties of haloalkanes and haloarenes.
In-depth analysis of key concepts and their applications in real-life scenarios.
Detailed explanation of important reactions such as the Wurtz reaction, Sandmeyer reaction, and Finkelstein reaction.
Solutions to a variety of problems to strengthen analytical and problem-solving abilities.
Step-by-step solutions for numerical problems and reaction mechanisms.
Important Study Materials for Class 12 Chemistry Chapter 6 Haloalkanes and Haloarenes
Students can access extra study materials on Haloalkanes and Haloarenes. These resources are available for download and offer additional support for your studies.
S.No | Related Links for Class 12 Chemistry Chapter 6: Haloalkanes and Haloarenes |
1. | |
2. | |
3. |
NCERT Solutions Class 12 Chemistry | Chapter-wise Links
Access Vedantu’s chapter-wise NCERT Chemistry Class 12 Solutions PDFs below for all other chapters.
S.No. | NCERT Solutions Class 12 Chemistry Chapter-wise List |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | Chapter 8 - Aldehydes, Ketones and Carboxylic Acids Solutions |
8 | |
9 |
NCERT Solutions Class 12 Chemistry - Related Links
S.No | Important Resources Links for Class 12 Chemistry |
1 | |
2 | |
3 | |
4 | |
5 |
Conclusion
NCERT Solutions for Class 12 Chemistry Chapter 6 - Haloalkanes and Haloarenes are invaluable resources for students. These solutions provide comprehensive explanations and step-by-step answers to the various problems and questions presented in the chapter. They help students understand the complex concepts of haloalkanes and haloarenes, including nomenclature, preparation, reactions, and their significance in organic chemistry. NCERT Solutions not only facilitate better comprehension but also aid in exam preparation, ensuring that students can tackle questions effectively. Furthermore, they align with the curriculum, making them an essential tool for academic success. Overall, NCERT Solutions for Class 12 Chemistry Chapter 6 serve as an indispensable aid for students aiming to excel in their chemistry studies, offering clarity and guidance in this critical area of organic chemistry.
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 6 Haloalkanes And Haloarenes - 2025-26
1. What are haloalkanes and haloarenes?
Haloalkanes are compounds where a halogen atom replaces a hydrogen atom in an aliphatic hydrocarbon. Haloarenes are compounds where a halogen is directly attached to a benzene ring. They are also known as alkyl halides and aryl halides, respectively.
2. Why are haloalkanes more reactive than haloarenes towards nucleophiles?
In haloarenes, the C-X bond has a partial double-bond character due to resonance, making it stronger. Also, the sp2 hybridised carbon in haloarenes is more electronegative, making it difficult for a nucleophile to approach and substitute the halogen atom.
3. What is the main difference between SN1 and SN2 reactions?
SN1 reactions are two-step processes that form a carbocation intermediate and are favoured by tertiary halides. SN2 reactions are single-step processes involving a transition state and are favoured by primary halides. Their reaction kinetics also differ.
4. What is Saytzeff's rule in elimination reactions?
Saytzeff's rule states that in a dehydrohalogenation reaction, the more highly substituted alkene (the one with more alkyl groups on the double-bonded carbons) will be the major product, as it is more stable.
5. How do NCERT Solutions for Class 12 Chemistry Chapter 6 help with exams?
NCERT Solutions provide step-by-step answers to all textbook questions, clarifying complex mechanisms and concepts. This helps in building a strong foundation and practicing the specific haloalkanes and haloarenes class 12 questions and answers frequently asked in exams.
6. Where can I find a free PDF for Haloalkanes and Haloarenes Class 12 NCERT Solutions?
You can download a free PDF of the NCERT Solutions for Class 12 Chemistry Haloalkanes and Haloarenes from the Vedantu website. This allows for easy offline access to all solved questions for practice and revision.
7. What topics are covered in the Class 12 Chemistry Chapter 6 NCERT Solutions?
The solutions cover all chapter topics, including nomenclature, physical and chemical properties, SN1 and SN2 mechanisms, elimination reactions, and reactions of haloarenes. All in-text and exercise problems are solved, ensuring complete coverage.
8. Why is chloroform stored in dark-coloured bottles?
Chloroform is stored in dark bottles because it slowly oxidises in the presence of light and air to form phosgene (carbonyl chloride), an extremely poisonous gas. The dark bottle prevents light from initiating this reaction, ensuring safety and purity.
9. Are both in-text and exercise questions solved in the Class 12 Chemistry Chapter 6 solutions?
Yes, the NCERT solution class 12 chemistry chapter 6 provides detailed answers for all questions. This includes every in-text question designed for conceptual checks and all end-of-chapter exercises that cover the entire topic comprehensively.
10. Why do haloarenes undergo electrophilic substitution reactions?
Although halogens are deactivating due to their -I effect, they are ortho, para-directing. This is because of the +R (resonance) effect, which increases electron density at the ortho and para positions, directing incoming electrophiles to these sites.


























