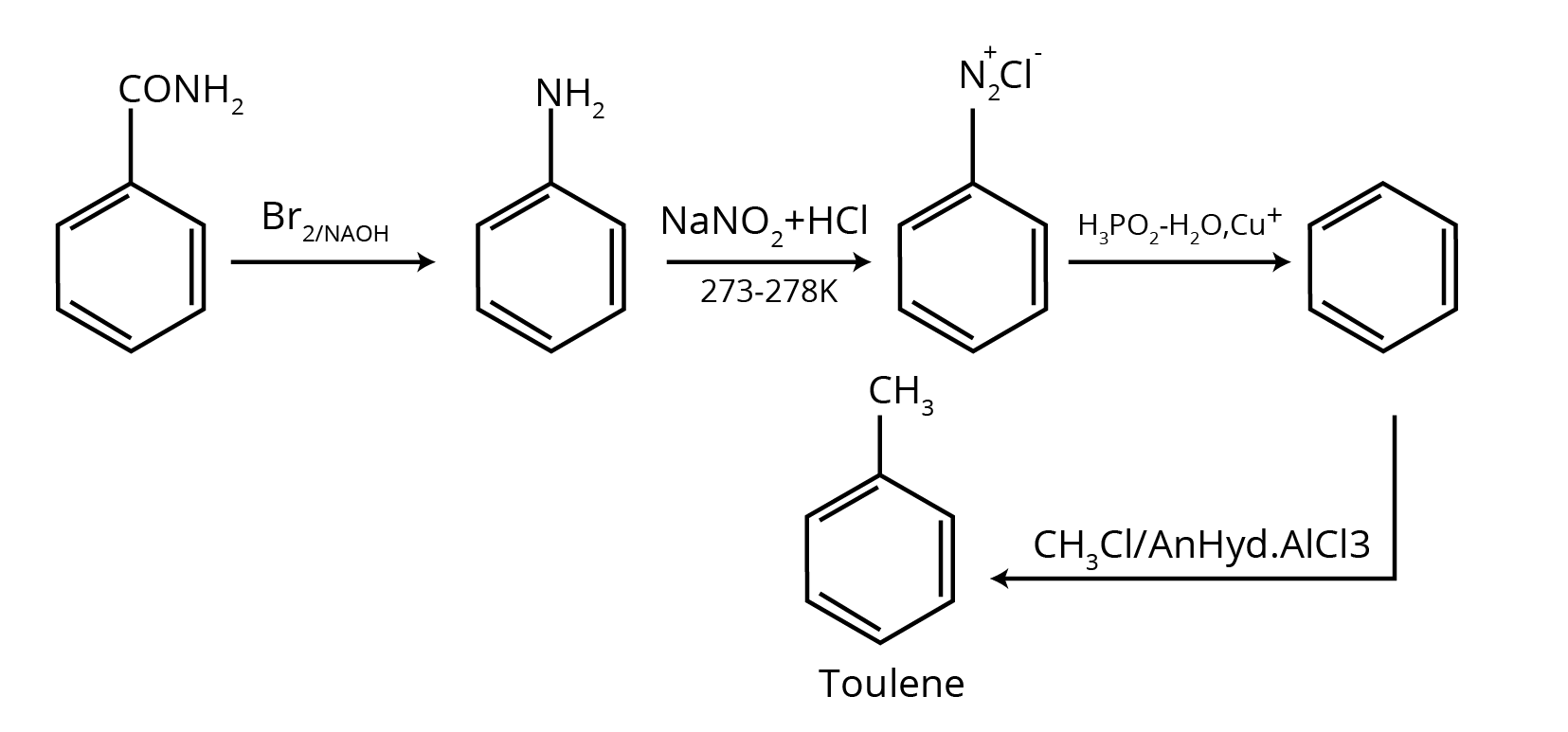
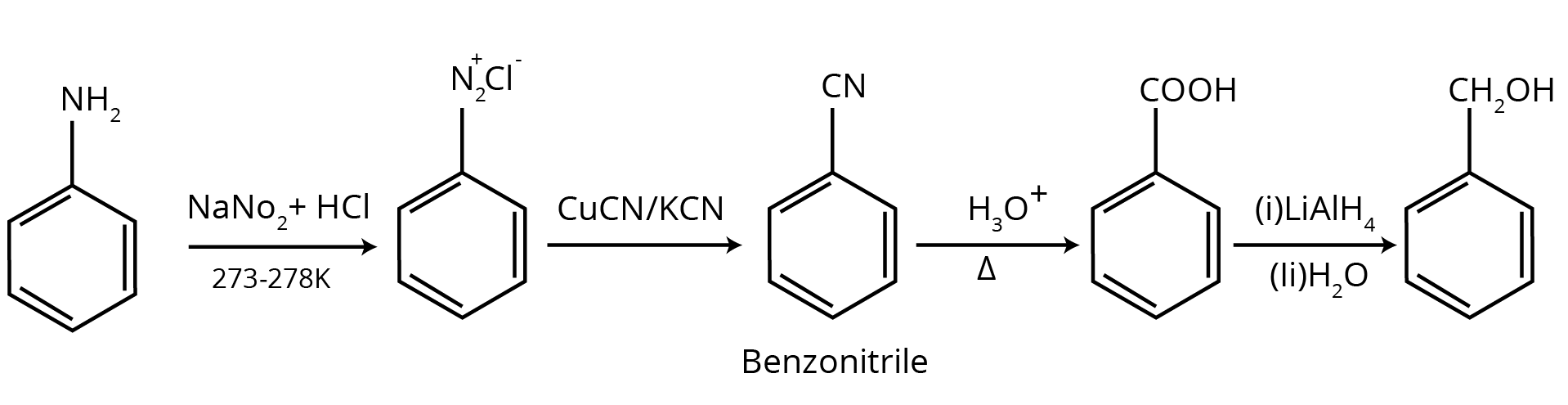
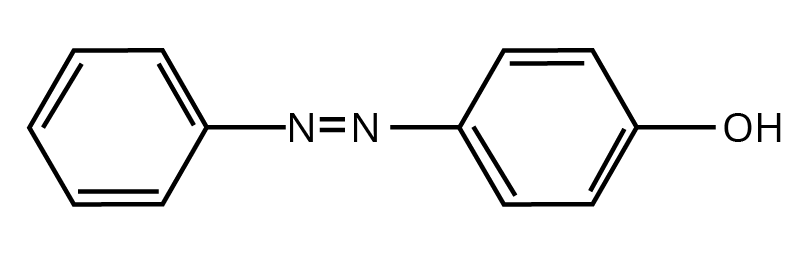
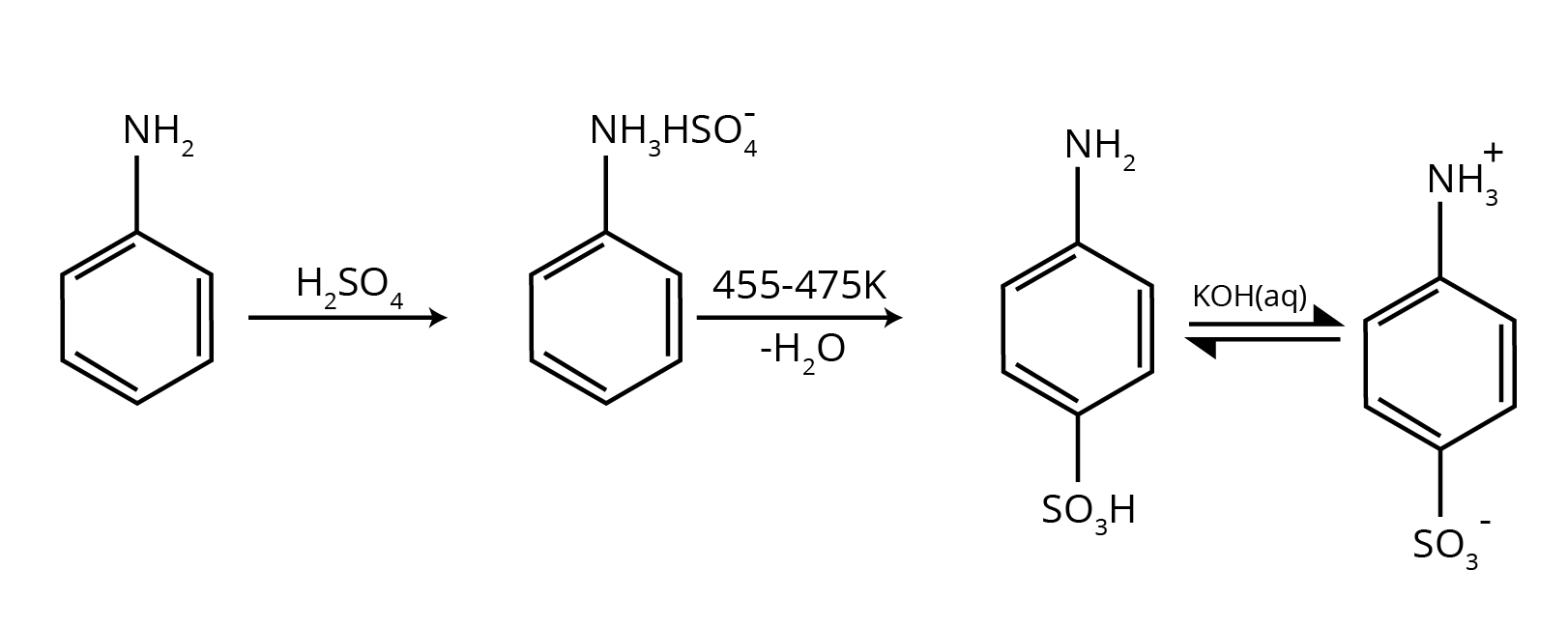
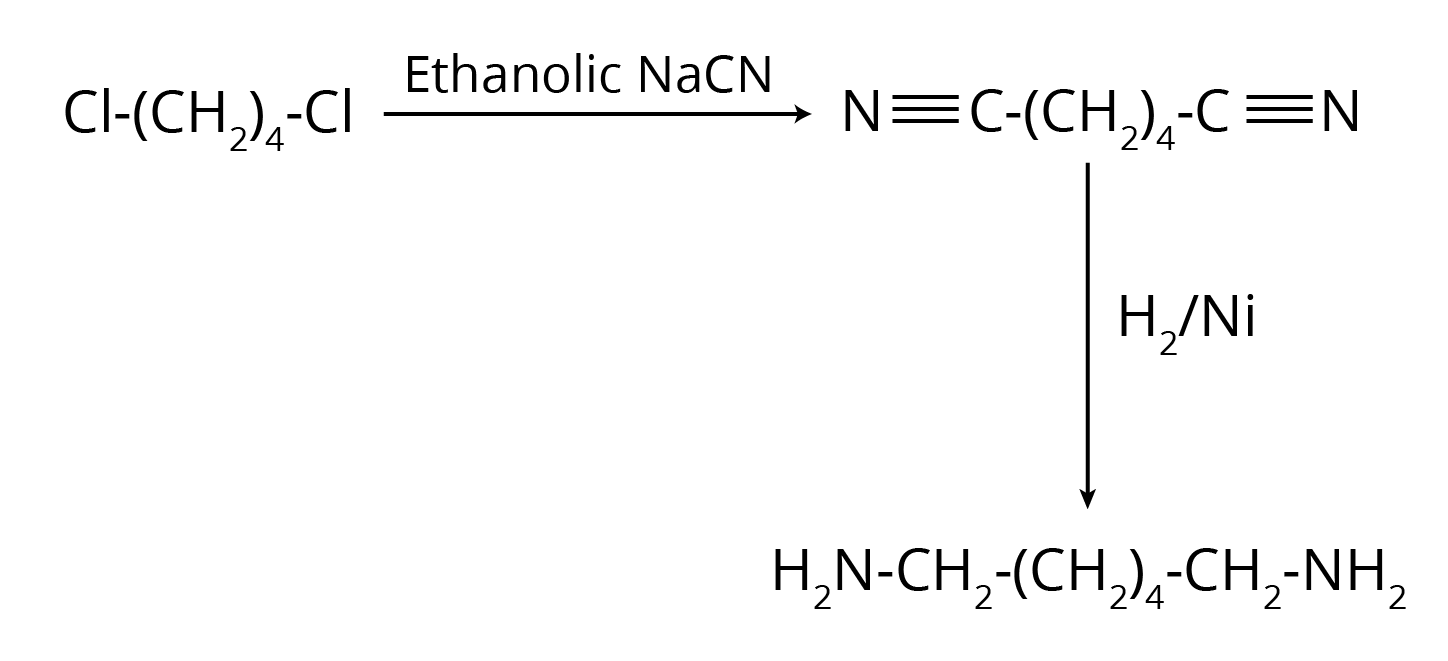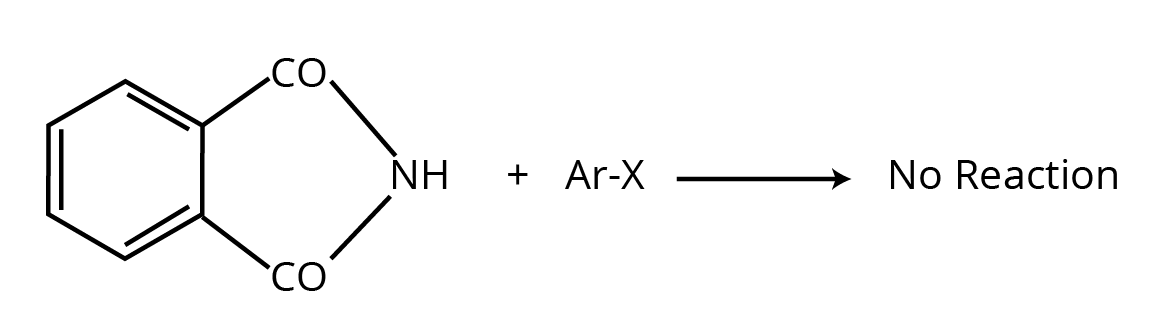How Can Class 12 Chemistry Chapter 9 NCERT Solutions Help With Amines Questions And Answers Class 12 Exam Preparation
FAQs on NCERT Solutions For Class 12 Chemistry Chapter 9 Amines (2025-26)
1. How do the NCERT Solutions for Chapter 9 provide a step-by-step method for solving chemical conversion problems involving amines?
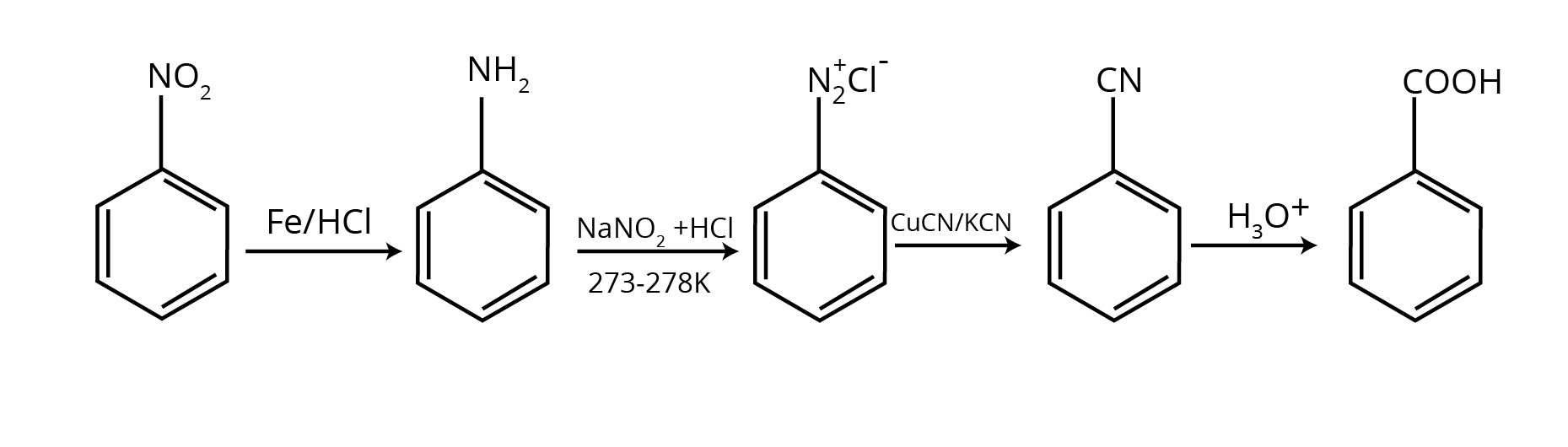
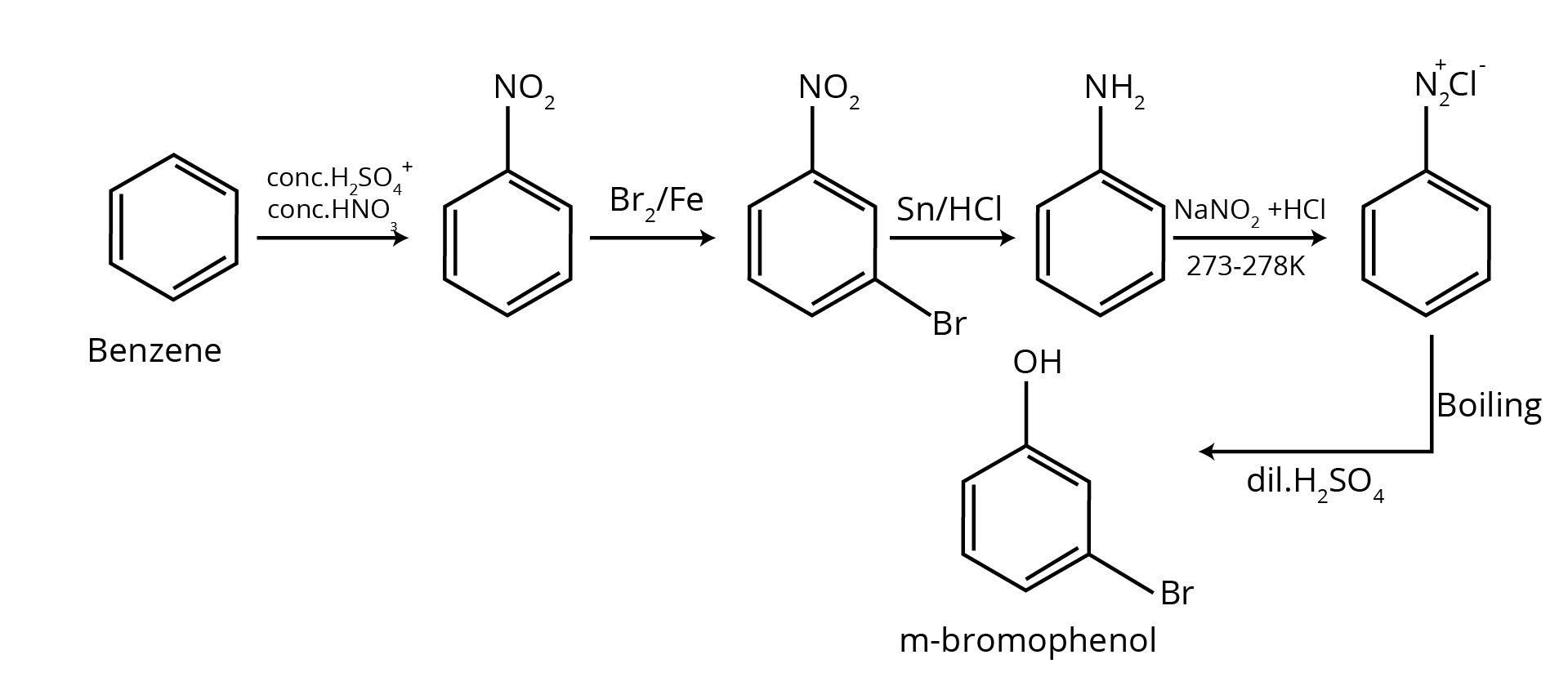
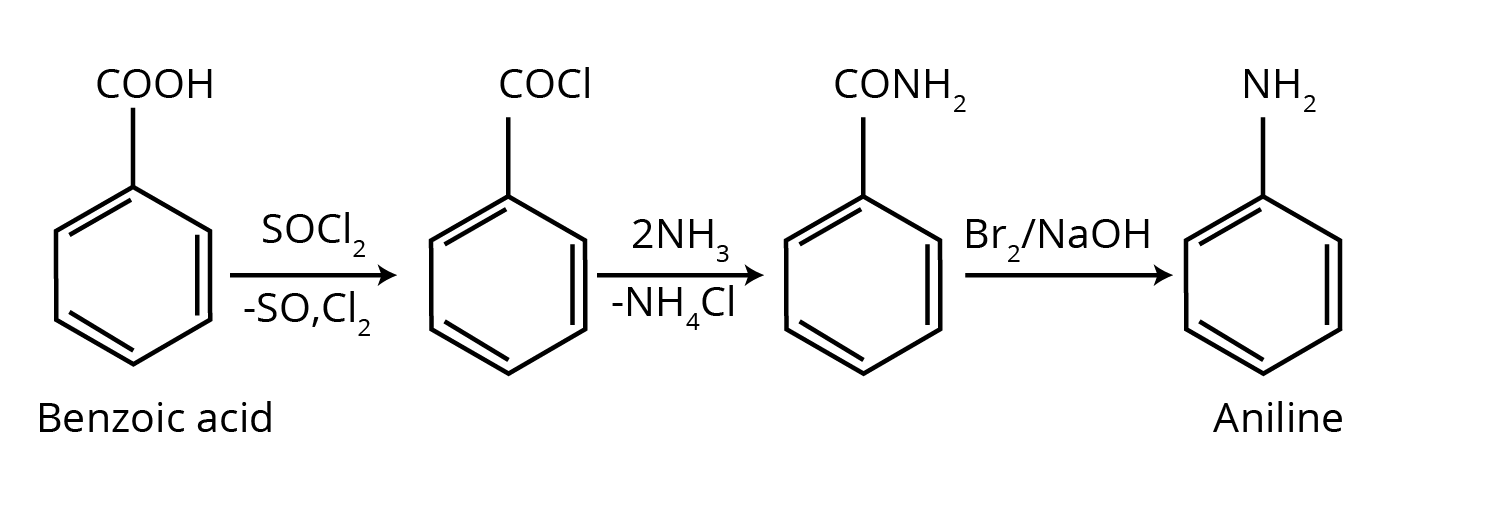
The NCERT Solutions for Class 12 Chemistry Chapter 9 break down conversion problems into a logical, stepwise process. The solutions guide you to:
- Identify the starting reactant and the final product to determine the change required (e.g., changing the number of carbon atoms).
- Outline a clear reaction pathway using appropriate reagents for each step.
- Write the balanced chemical equation for every stage of the conversion, ensuring you are following the correct NCERT methodology.
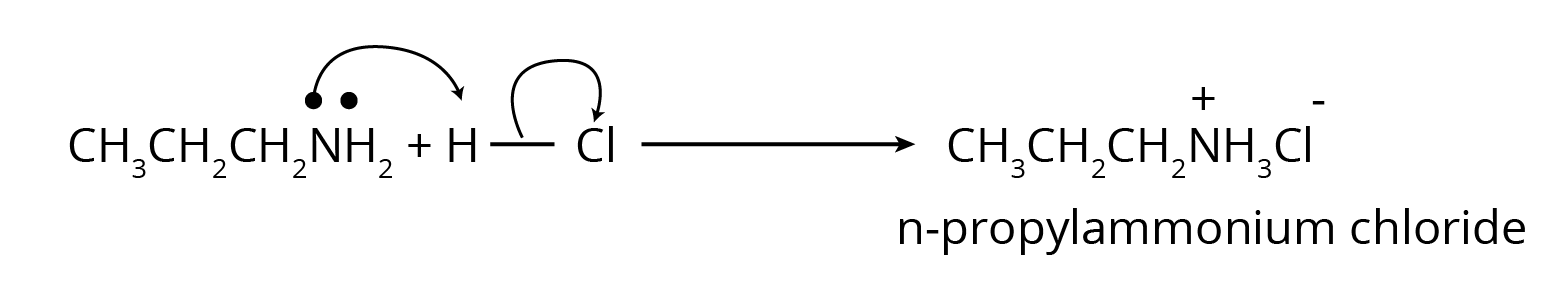
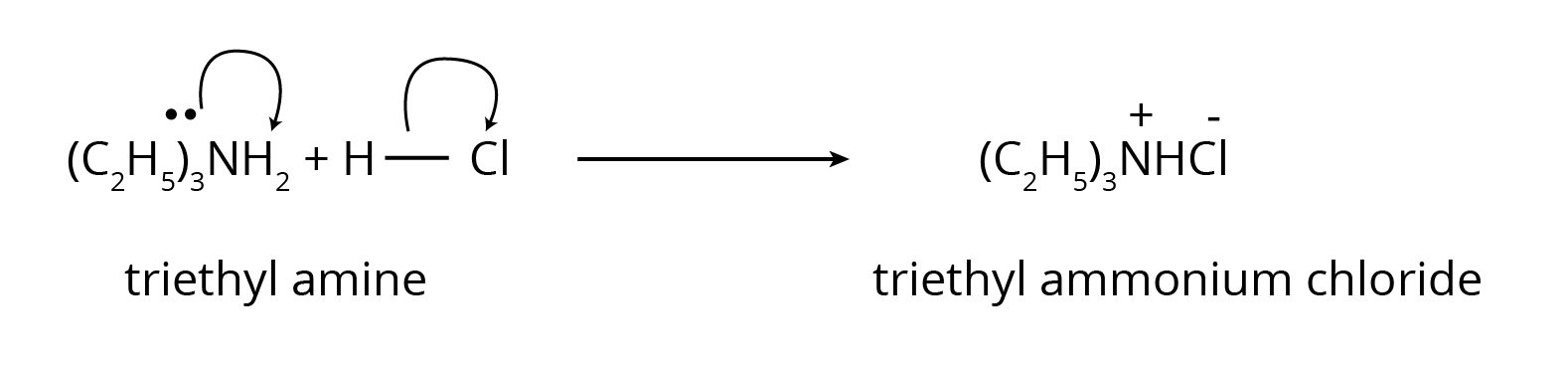
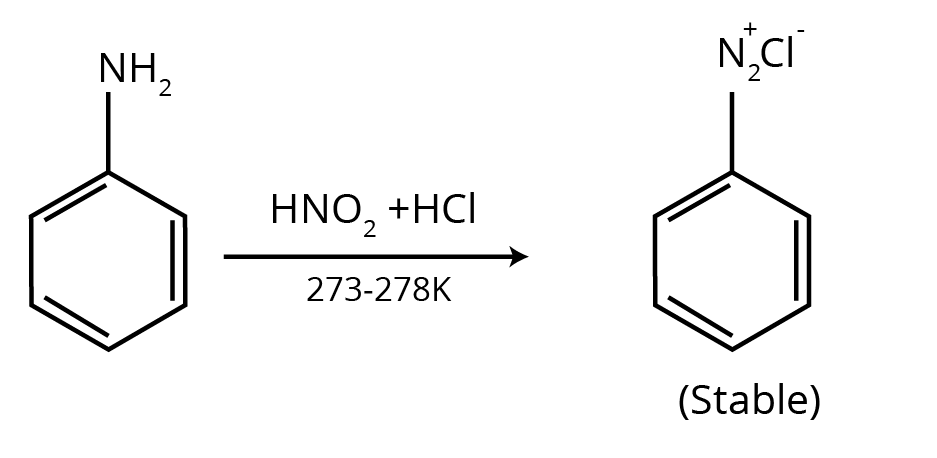
2. What is the correct way to present the answer for a chemical test to distinguish between two amines, as per the CBSE pattern?
To correctly answer questions on distinguishing between amines (e.g., primary vs. secondary), the NCERT solutions demonstrate a precise format:
- Name the specific test used (e.g., Carbylamine test or Hinsberg's test).
- Write the complete chemical reaction for the amine that gives a positive result, mentioning the observable change (e.g., formation of a precipitate, unpleasant odour, or colour change).
- For the other amine, clearly write the reaction showing a negative result or stating 'No reaction'.
3. How should I approach solving questions on arranging amines in increasing or decreasing order of their basic strength from the NCERT exercise?
The NCERT Solutions for Chapter 9 show that simply writing the final order is insufficient. A complete answer requires a stepwise explanation:
1. Identify all the factors affecting basicity for the given amines, such as the +I (inductive) effect of alkyl groups, the -R (resonance) effect in aromatic amines, and steric hindrance.
2. Compare the amines based on these effects to determine the availability of the lone pair of electrons on the nitrogen atom.
3. Write the final order of basic strength with a clear justification for the position of each compound in the series.
4. Are the solved answers in the NCERT Solutions for Class 12 Amines updated for the latest 2025-26 CBSE syllabus?
Yes, the NCERT Solutions for Class 12 Chemistry Chapter 9 (Amines) are fully updated to align with the latest CBSE syllabus for the 2025-26 academic year. All the in-text and exercise questions are solved following the official NCERT guidelines and marking schemes, making them a reliable resource for board exam preparation.
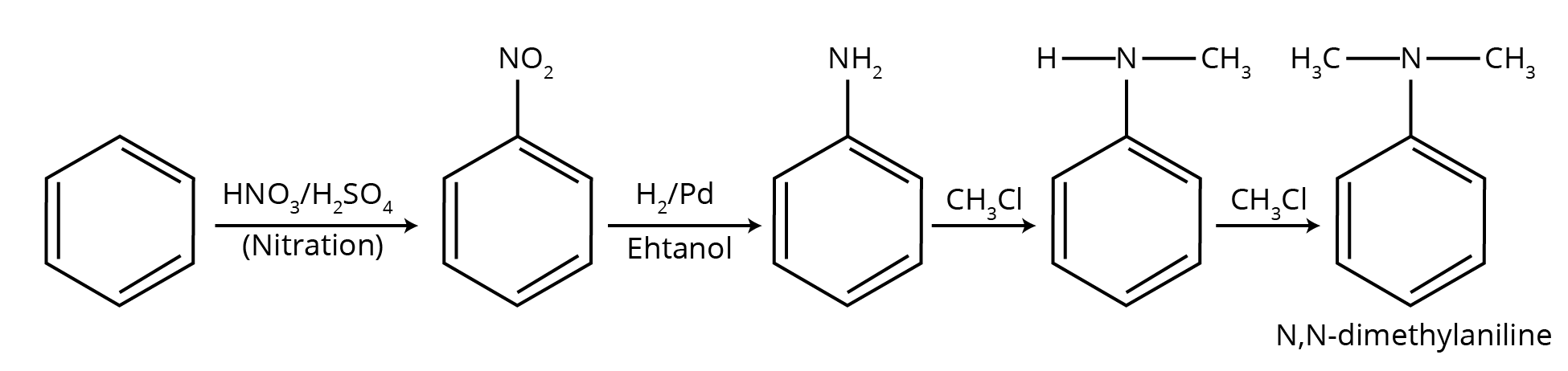
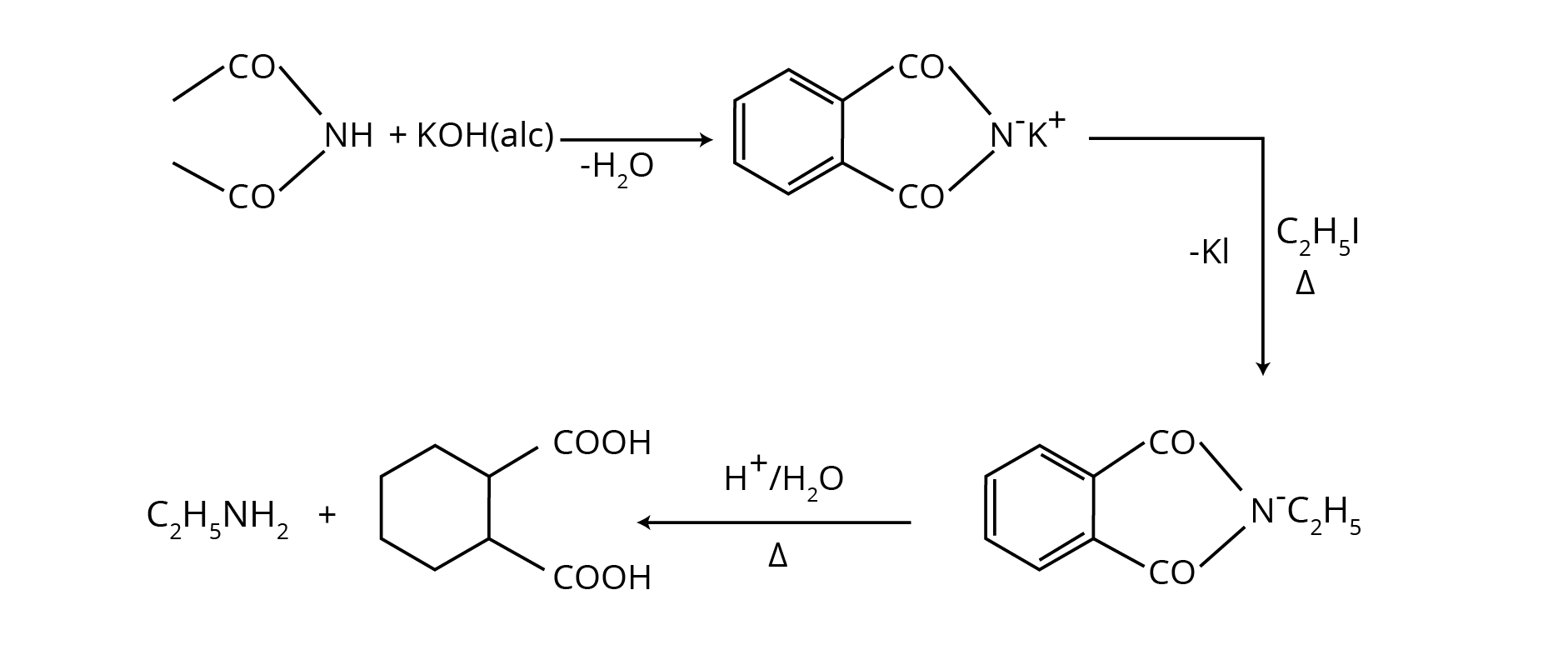
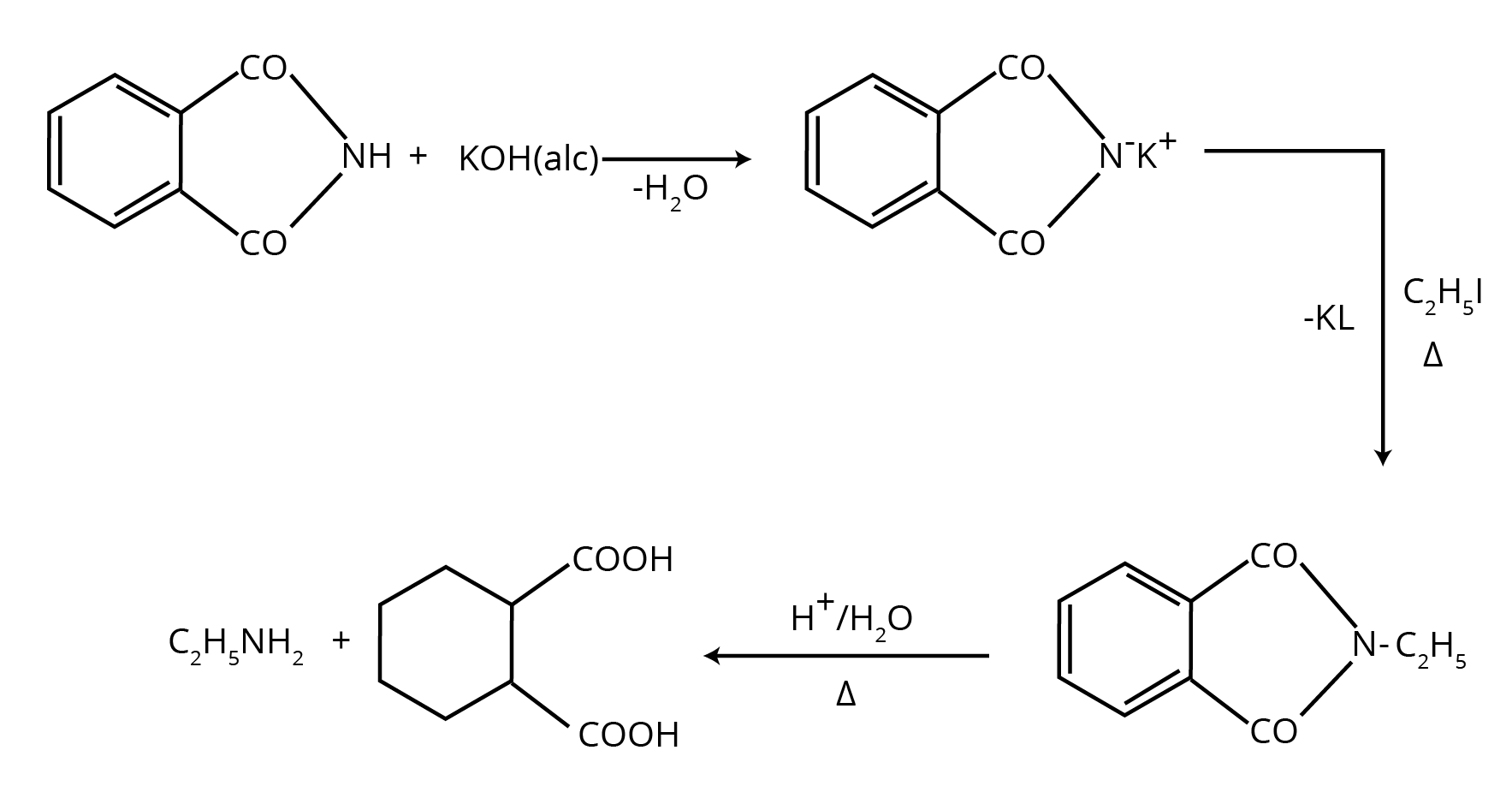
5. How do the NCERT solutions explain important named reactions like the Hofmann Bromamide reaction or Gabriel Phthalimide synthesis?
The solutions provide a comprehensive, step-by-step explanation for all named reactions in the Amines chapter. For each reaction, you will find:
- A clear definition or statement of the reaction.
- The general chemical equation showing the reactants, reagents, and products.
- A specific, solved example from the NCERT textbook to illustrate the reaction's application.
- Explanation of the reaction's significance, for instance, why Gabriel synthesis is preferred for preparing pure primary amines.
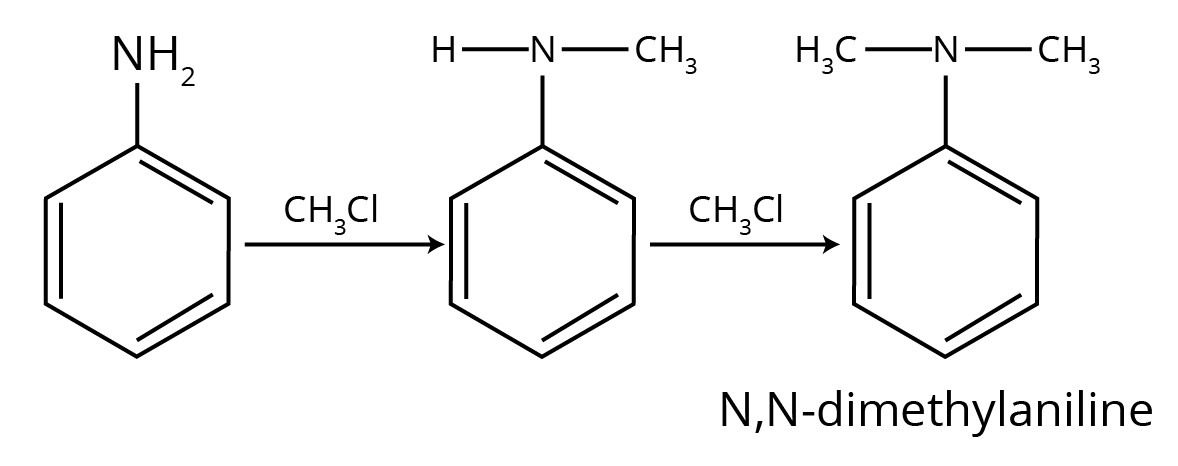
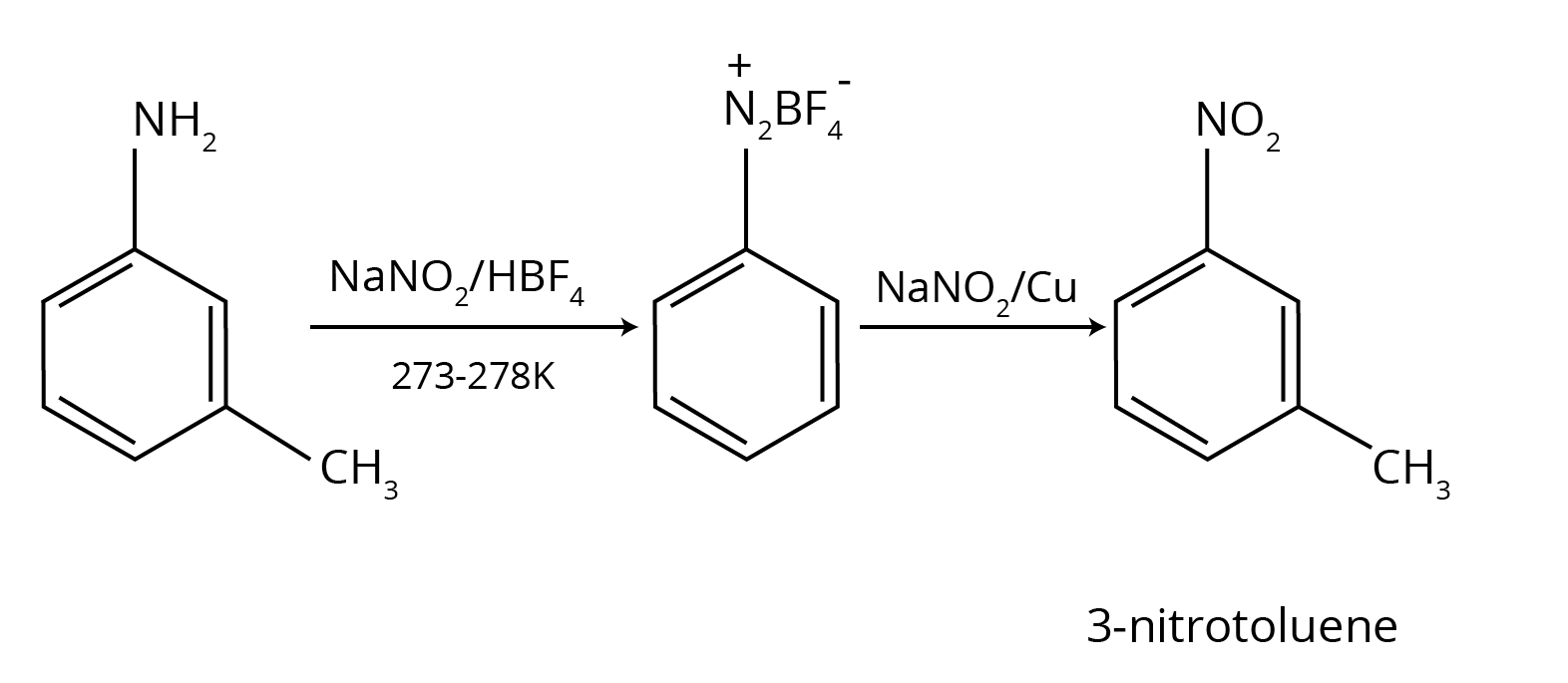
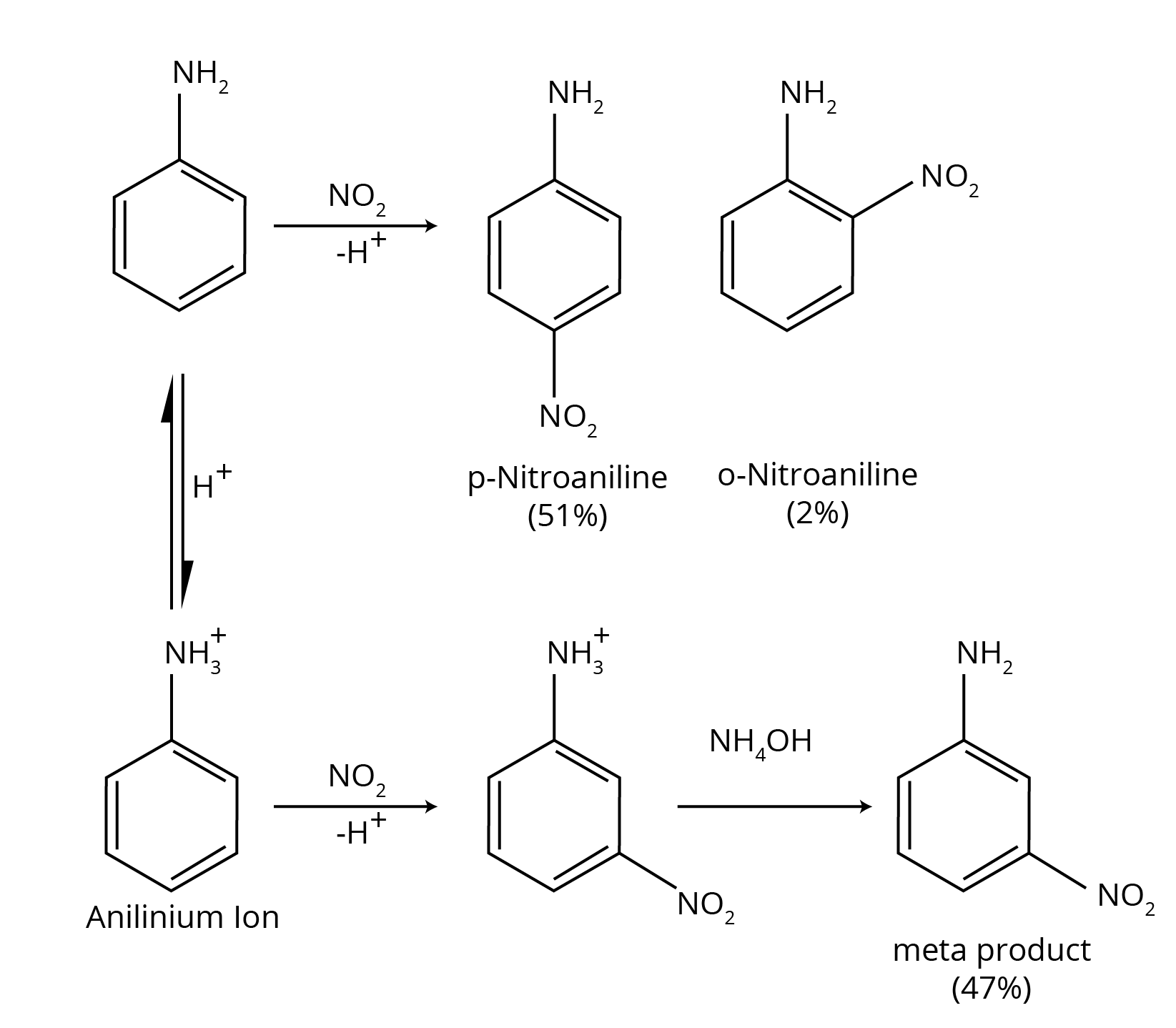
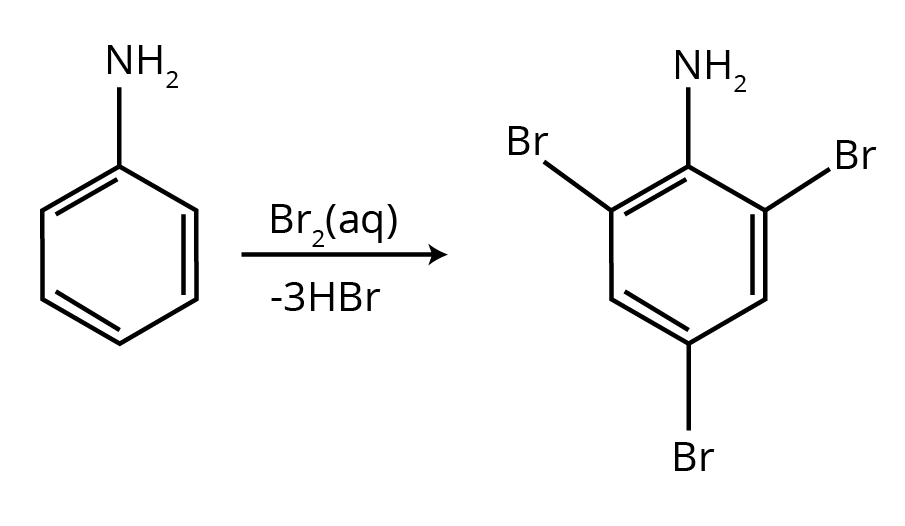
6. How do the NCERT Solutions for Amines clarify why aniline gives a substantial amount of m-nitroaniline during nitration?
This is a classic conceptual question, and the NCERT solutions provide a detailed, stepwise explanation. Nitration is carried out in a strongly acidic medium. The solutions explain that in this medium, a significant portion of aniline is protonated to form the anilinium ion (-NH₃⁺). While the amino group (-NH₂) is ortho, para-directing, the anilinium ion is a meta-directing group. Therefore, the nitration of this mixture results in both o/p-nitroaniline and a substantial amount of m-nitroaniline.
7. What are common mistakes to avoid when solving problems from the NCERT Amines chapter?
Students often make mistakes in a few key areas, which the step-by-step NCERT solutions help prevent:
- Basicity Order: Forgetting to consider steric hindrance in tertiary amines or the effect of the solvent.
- Chemical Tests: Not writing the specific observable change (e.g., colour, smell).
- Conversions: Using an incorrect reagent or not balancing the atoms in multi-step reactions.
- Nomenclature: Incorrectly naming substituted anilines or secondary/tertiary amines.
8. How should I use the NCERT solutions to answer why Gabriel Phthalimide synthesis is not used for preparing aromatic primary amines?
The NCERT solutions guide you to structure this reasoning-based answer correctly. First, state the core reason: this synthesis involves a nucleophilic substitution reaction where the phthalimide anion attacks an alkyl halide. The solutions clarify that aryl halides (like chlorobenzene) are much less reactive towards nucleophilic substitution due to the partial double-bond character between the carbon and halogen atom caused by resonance. Therefore, the phthalimide anion cannot displace the halide from the benzene ring, making this method unsuitable for preparing aniline.
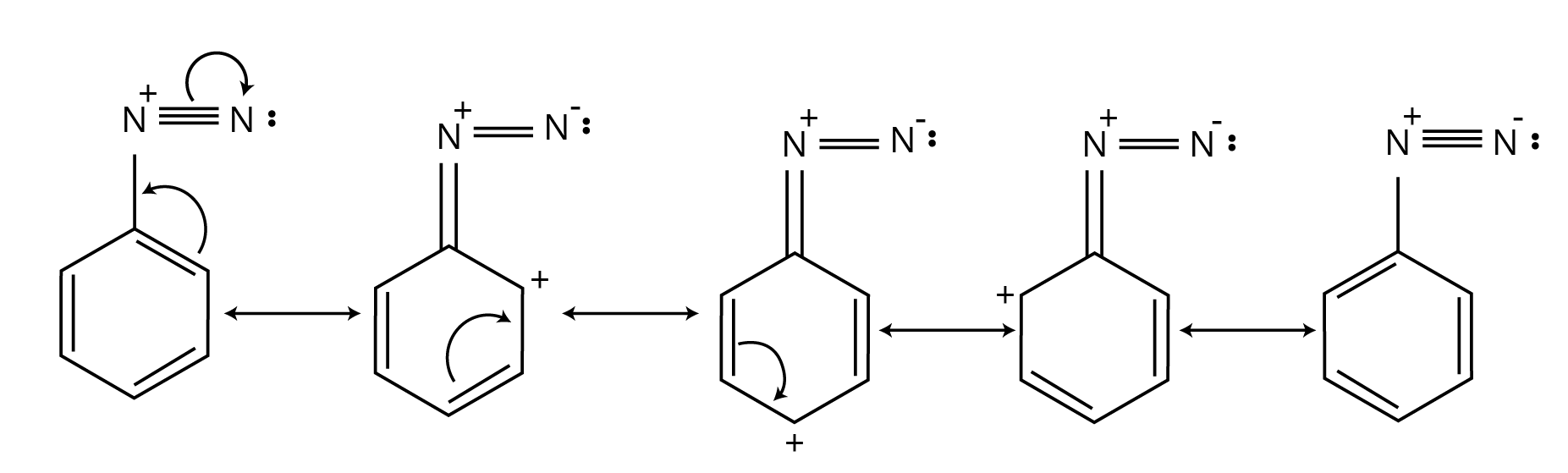
9. For questions requiring a reaction mechanism in Chapter 9, what is the expected level of detail according to the NCERT solutions?
When a mechanism is explicitly asked for, the NCERT solutions show that you must provide a clear, step-by-step pathway. This includes:
- Showing the movement of electrons using curved arrows.
- Drawing the structures of all intermediates and transition states.
- Indicating the attack of the electrophile or nucleophile in each step.
10. Why is it important to explain the underlying principles, like inductive and resonance effects, when solving questions about the pKb value of amines?
The NCERT solutions emphasize explaining these principles because the pKb value is inversely related to basic strength. A lower pKb means a stronger base. To justify the pKb order, you must explain *why* one amine is more or less basic than another. This involves discussing:
- Electronic effects: How inductive (+I) and resonance (-R) effects alter the electron density on the nitrogen atom.
- Steric factors: How bulky groups can hinder the approach of a proton.