




What does Rate of Reaction refers?
In a chemical reaction, the rate of reaction or the reaction kinetics refers to how quickly the products are generated from the reactants. The reaction rate of cellulose combustion in fire, for example, is extremely high, and the reaction is finished in less than a second.
The rate of reaction, also known as the reaction rate, is the rate at which reactants become products. Some chemical reactions occur almost instantly, while others take a long period to achieve their final equilibrium.
The rate constant, also known as the specific rate constant, is the proportionality constant in the equation that represents the connection between a chemical reaction's pace and the concentrations of the reacting chemicals.
Effect of Pressure on Rate of Reaction
For gases, partial pressure is another way to indicate concentration. The number of collisions rises as the partial pressures of gases increase. As a result, the rate of reactions involving gaseous reactants rises as partial pressures rise. It has no effect, however, on reactions involving reactants in liquid or solid phases. It is wise to note that the partial pressures of reactants can be raised by raising the total system pressure. When a consistent amount of inert gas or a non-reacting gas is introduced to the reaction mixture, the partial pressure does not rise.
When the pressure of gaseous reactants is raised, the number of reactant particles per volume increases. Because there will be more collisions, the reaction rate will increase. The higher the pressure of the reactant, the quicker the reaction will occur.
Effect of Temperature on Rate of Reaction
The pace of reaction increases as the temperature rises. For many reactions occurring at or near room temperature, the rate of reaction roughly doubles for every 10°C increase in temperature.
Only when particles collide can they react. When the temperature is raised, the particles travel quicker and collide more frequently. This will increase the rate of reaction.
Some reactions, like precipitation, involve the coming together of ions in solution to form an insoluble solid, or the interaction between hydrogen ions from an acid and hydroxide ions from an alkali in solution. As a result, heating one of them will have no discernible effect on the pace of the reaction.
With the increase in temperature, the rate of the reaction and the rate constant increases.
Nature of reactants
The rate of a reaction is determined by the type of bonding in the reactants. Ionic chemicals often react quicker than covalent ones. Because they merely entail the exchange of ions that were already separated in aqueous solutions during their dissolution, interactions between ionic compounds in water occur relatively quickly.
When the AgNO3 solution is introduced to NaCl solution, AgCl precipitates out instantly.
$\mathrm{AgNO}_{3(\text { aq })}+\mathrm{NaCl}_{(\text {aq })} \rightarrow \mathrm{AgCl}_{(\mathrm{s})} \downarrow+\mathrm{NaNO}_{3(\text { aq })}$
This process is incredibly quick since it simply requires the exchange of ions, as seen below:
$\mathrm{Ag}^{+}+\mathrm{NO}_{3}^{+}+\mathrm{Na}^{+}{ }_{(\mathrm{aq})}+\mathrm{Cl}^{-}{ }_{(\mathrm{aq})} \rightarrow \mathrm{AgCl}_{(\mathrm{s})} \downarrow+\mathrm{Na}_{(\mathrm{aq})}^{+}+\mathrm{NO}_{3}^{-}$
Covalent compound reactions, on the other hand, are slow because they need the energy to dissolve existing bonds. Example, Acetic acid esterification takes time because bond breaking requires energy.
Effect of Catalyst
A catalyst is a chemical that does not participate in the reaction but accelerates the pace of the reaction in some way. Catalysts open up new paths for the reaction. The reactants take only a little amount of energy to transform into products. Some catalysts boost the pace of reaction in a variety of reactions, but others, such as enzymes, which are found in human bodies, are specialised for a single reaction or kind of reactant molecule. As a result, a catalyst accelerates the pace of a chemical process.
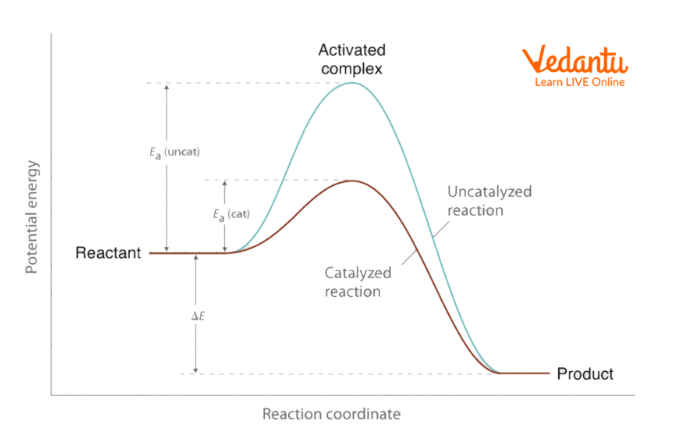
Effect of Catalyst on Reaction Rate
The rate constant depends on the catalyst. At any given temperature, the rate constant for the catalysed reaction will be larger.
Summary
The rate of the reaction determines how quickly the chemical reaction occurs. The speed of the response is affected by a number of variables. It is frequently stated in terms of the concentration of a product created in a unit of time or the concentration of a reactant consumed in a unit of time. There are different factors that talk about the rate of reaction.
Concentration: The rate of reaction rises as the concentration of the reactants increases.
Reactant nature: The condition, reactivity, or solution type of the reactants all impact the rate of the reaction.
Pressure: Increasing the pressure on a responding gas reaction accelerates the reaction. The pace of a reaction involving just solids or liquids is unaffected by changing the pressure.
Solid surface area: The rate of response rises as the exposed surface area increases.
FAQs on Factors Affecting Rate of Reaction for JEE
1. How can you figure out the rate of reaction?
The question here concerns the rate of the chemical reaction. We know that in a chemical reaction, a reactant undergoes change under appropriate reaction circumstances, yielding the products. We need to know the rate or speed at which the chemical reaction is taking place. We may deduce from this statement that everything that rate is reliant on is the concentration of the reactants or the concentration of the products. As a result, if we notice the concentration terms and the time required, we may deduce that we can measure the rate of reaction.
2. What are some examples of everyday scenarios where the impact of surface area in a chemical reaction is used?
When washing garments, the surface area of the washing liquid is expanded by diluting it in a big bucket and enough room is provided for the reaction to occur. This facilitates the chemical process. If utilising a washing machine, the first soaking and spinning are done by the machine when the washing powder is introduced and a tap is opened to dissolve the powder. When cooking vegetables in the kitchen, the vegetables are distributed and then covered with a lid to allow the boiling heat and other chemical processes to occur uniformly.
























