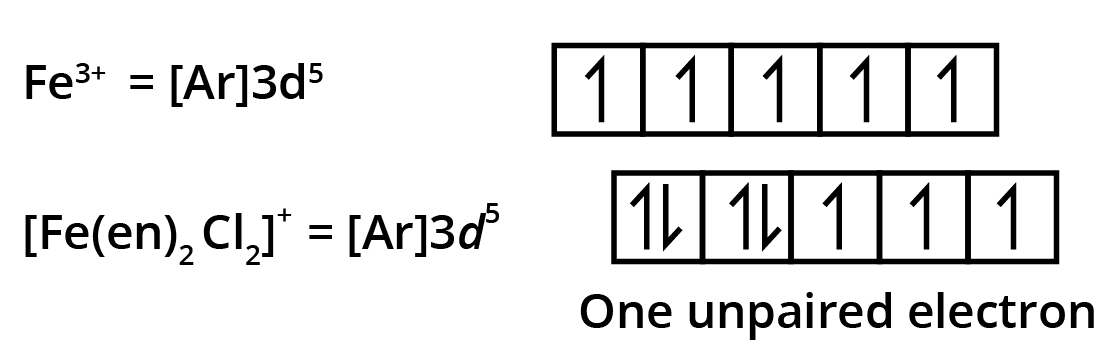Coordination Compounds Class 12 Extra Questions and Answers Free PDF Download
FAQs on CBSE Important Questions for Class 12 Chemistry Coordination Compounds - 2025-26
1. What are the most important topics in CBSE Class 12 Chemistry Chapter 5 – Coordination Compounds for the 2025–26 exam?
- Nomenclature of Coordination Compounds
- Werner’s Theory and Valence Bond Theory (VBT)
- Crystal Field Theory (CFT) and splitting patterns
- Types of ligands, coordination number, and chelation
- Isomerism: Geometrical, Optical, and Linkage Isomerism
- Applications of coordination compounds (including biological roles)
2. How are important questions for Coordination Compounds in Class 12 Chemistry selected as per latest CBSE trends?
Important questions are curated based on:
- Repetition in previous 5–7 years’ CBSE board papers
- Appearances in sample papers and official CBSE model sets
- Covering hallmark concepts (e.g., naming, isomerism, electronic structure)
- Problems requiring application—especially calculation-based (magnetic moment, oxidation state, splitting patterns)
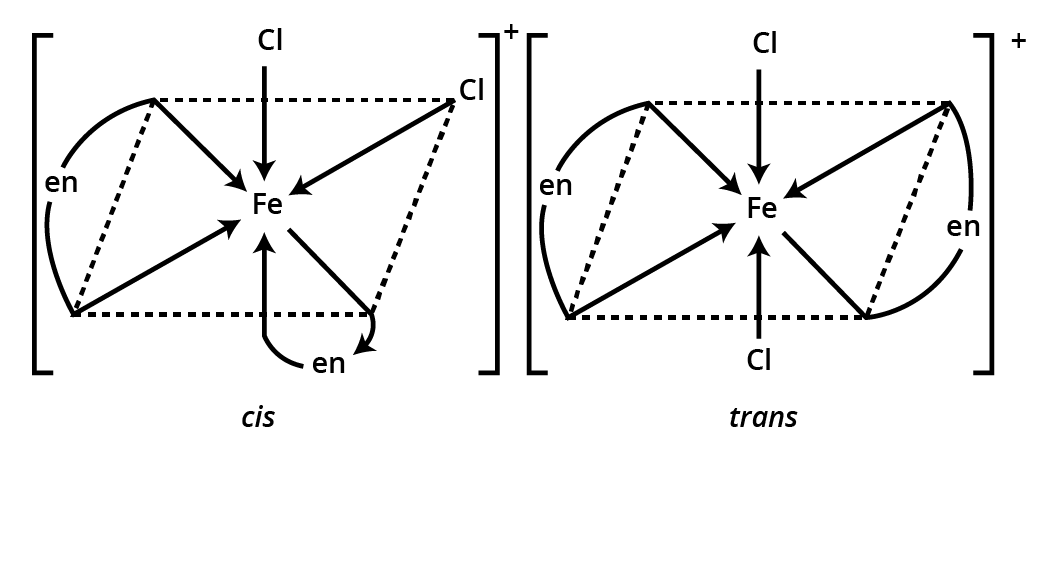
3. Which types of isomerism are frequently tested in Coordination Compounds Class 12 important questions?
The most examined types include:
- Geometrical isomerism (cis-trans, fac-mer in octahedral and square planar complexes)
- Optical isomerism (especially in complexes with bidentate ligands like en and oxalate)
- Linkage and ionization isomerism
4. What are the marking scheme and weightage for Coordination Compounds important questions in the CBSE 2025–26 exam?
- Short answer (1–2 marks): Definitions, formulas, simple nomenclature, and direct factuals
- Short answer-II (3 marks): Application-based Qs (isomerism, calculation of oxidation state, hybridization)
- Long answer (5 marks): HOTS, detailed comparison, structural illustrations, multi-step explanations (e.g., electronic structure + geometry + paramagnetism)
5. How can students avoid common mistakes in attempting Coordination Compounds important questions?
- Always write full IUPAC names per official rules—no short forms.
- Show calculation steps for oxidation state, magnetic moments, and drawing geometrical isomers.
- Differ clearly between types of isomerism—especially linkage, ionization, and coordination isomers.
- Never confuse chelating ligands with simple bidentate ligands—justify your example.
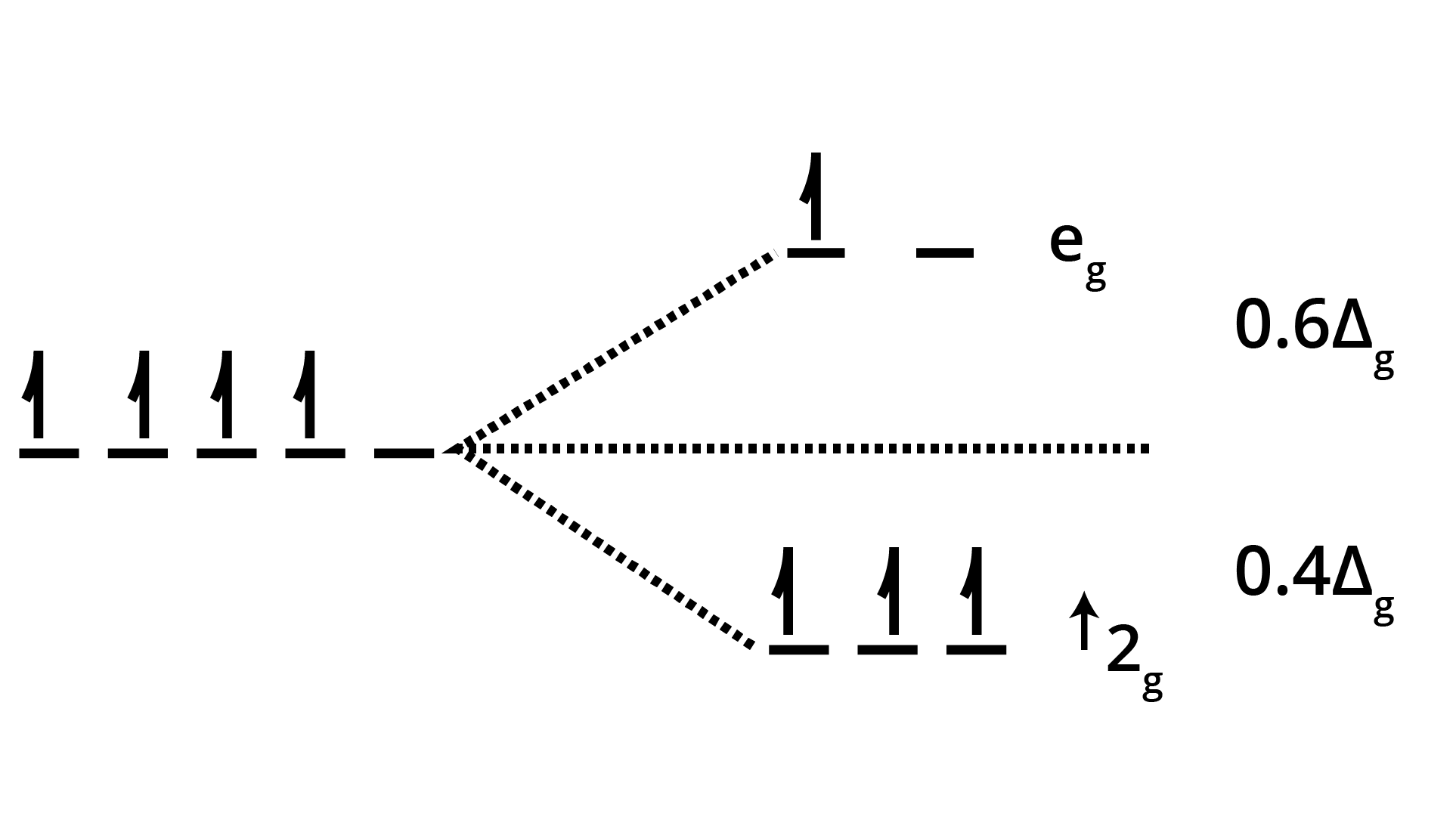
6. What is a frequently asked HOTS question on Crystal Field Theory in Class 12 Coordination Compounds?
The question often focuses on predicting magnetic behaviour and geometry based on ligand strength:
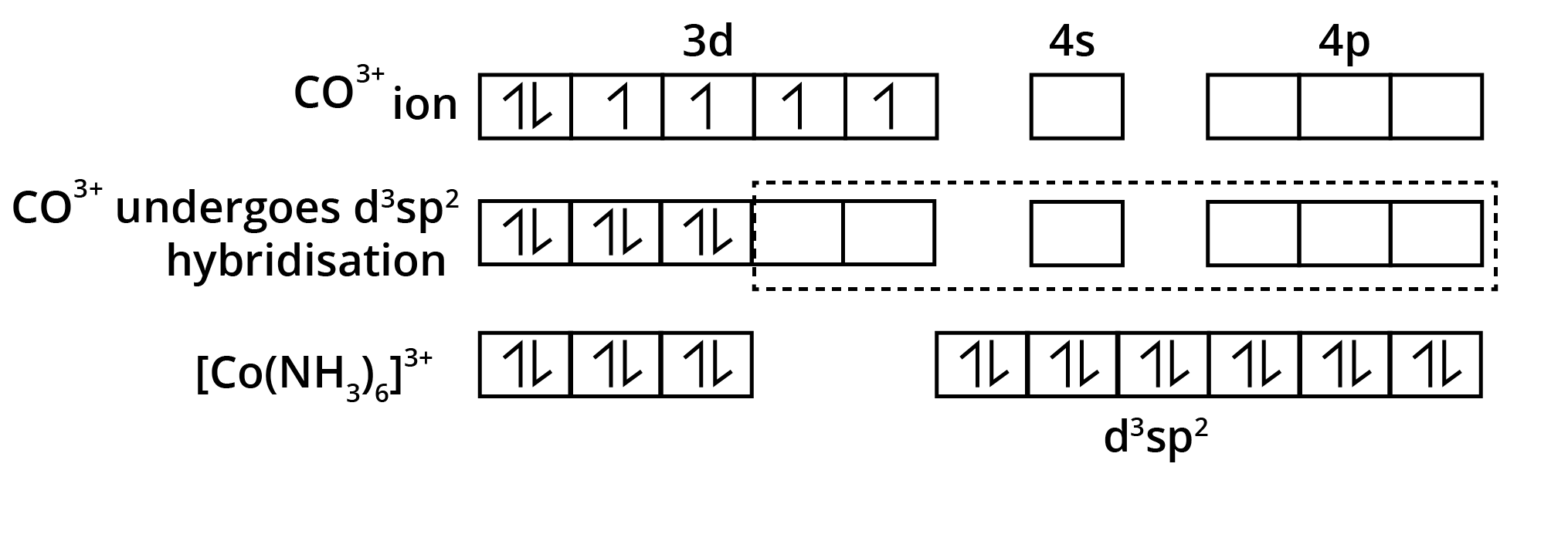
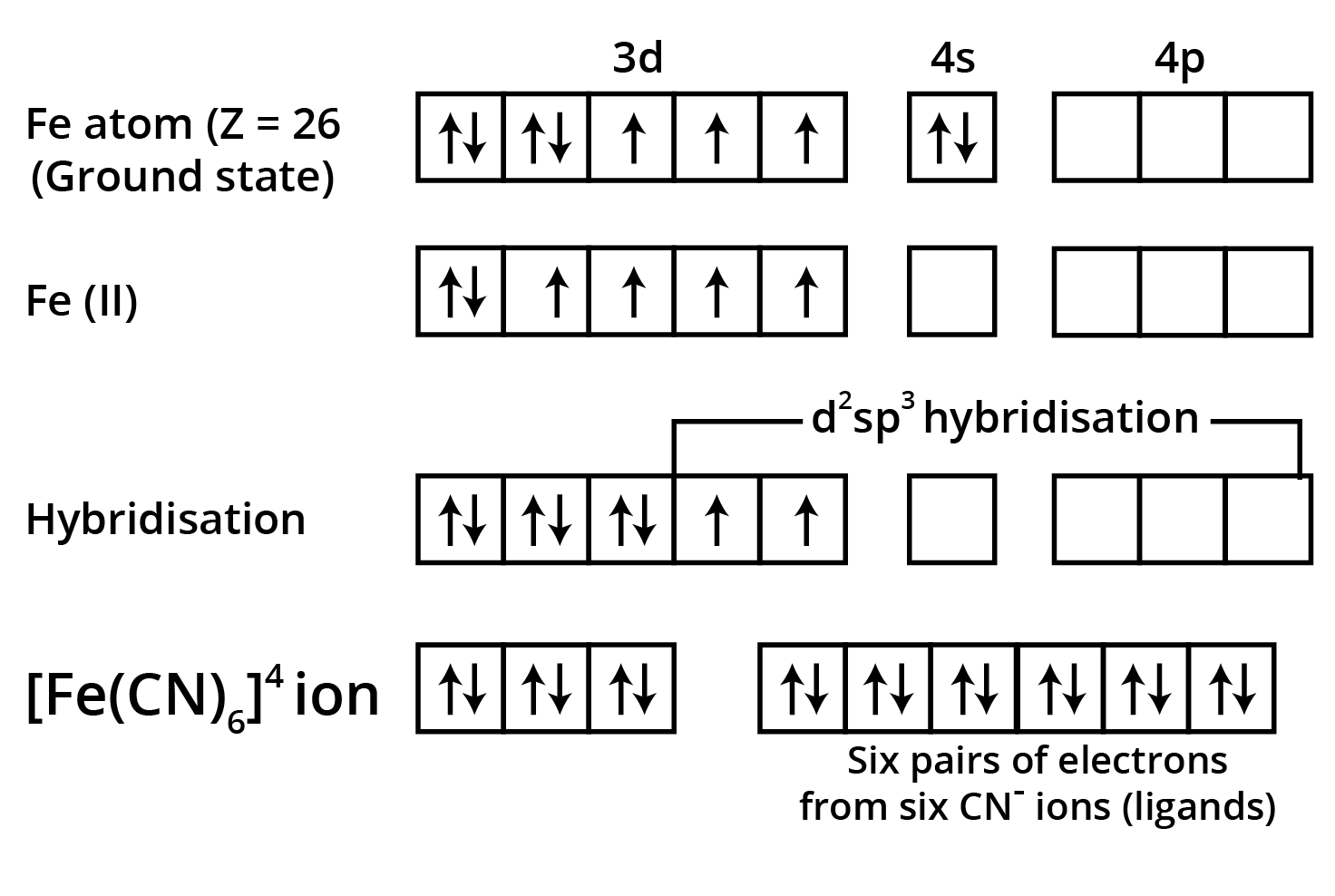
"Explain, with electronic configurations, why [Fe(CN)6]4– is diamagnetic while [FeF6]3– is paramagnetic, although both are octahedral."
Marks are awarded for correct splitting diagram, electron filling, and tying theory to observed magnetism.
7. What kind of numerical problems are included among important questions for Coordination Compounds Class 12?
Expected numericals include:
- Calculation of overall stability/dissociation constants from data
- Determining magnetic moment using number of unpaired electrons
- Applying freezing point depression data to deduce complex formula
8. Why are chelating ligands and their effects on complex stability considered an important exam topic?
- Chelating ligands form stable ring structures with metal ions, increasing complex stability due to the chelate effect.
- This concept is frequently explored in application questions, asking students to compare the relative stability of complexes formed with chelating versus non-chelating ligands.
9. What are some difficult conceptual traps for students in important questions on Coordination Compounds?
- Mixing up oxidation number with coordination number
- Confusing different types of isomerism, especially linkage vs. ionization
- Neglecting ligand field strength when predicting electron pairing/geometries
- Incorrectly counting donor atoms in polydentate ligands
10. How do examiners expect students to approach 5-mark Coordination Compounds questions involving comparison or reasoning?
Students should:
- State differences/comparisons point-wise (at least two major and two minor aspects)
- Support reasoning with correct structures, electron configurations, and theoretical background
- Conclude with application/context as per CBSE marking guidelines
11. What are the key factors that determine the colour of coordination compounds, a common important question?
- Nature of metal ion (oxidation state)
- Type of ligands attached (strength/field splitting ability)
- Geometry of the complex (octahedral, tetrahedral)
12. How are Coordination Compounds important in real-life applications, as typically asked in CBSE board exams?
Common real-life applications asked include:
- Biological functions (e.g., haemoglobin, chlorophyll, vitamin B12)
- Chemotherapy agents (cis-platin)
- Water purification and hardness estimation (EDTA titration)
- Electroplating and photographic processes
13. What are frequently missed but important differences between double salts and coordination compounds expected in Class 12 exams?
- Double salts dissociate completely in water, while coordination compounds retain their identity.
- Double salts give all ions in solution, complexes give characteristic group reactions.
- Example: Mohr’s salt (double salt) vs. Potassium ferrocyanide (coordination compound).
14. What is the best approach for answering mechanism-based HOTS questions in Coordination Compounds?
- Start by identifying the central atom, ligands (type and denticity), and oxidation state.
- Draw the structure if asked (showing geometry/isomerism).
- Use knowledge of VBT, CFT, and real-world examples to justify your answer.
- Always connect theory to observed property (colour, magnetism, stability).

























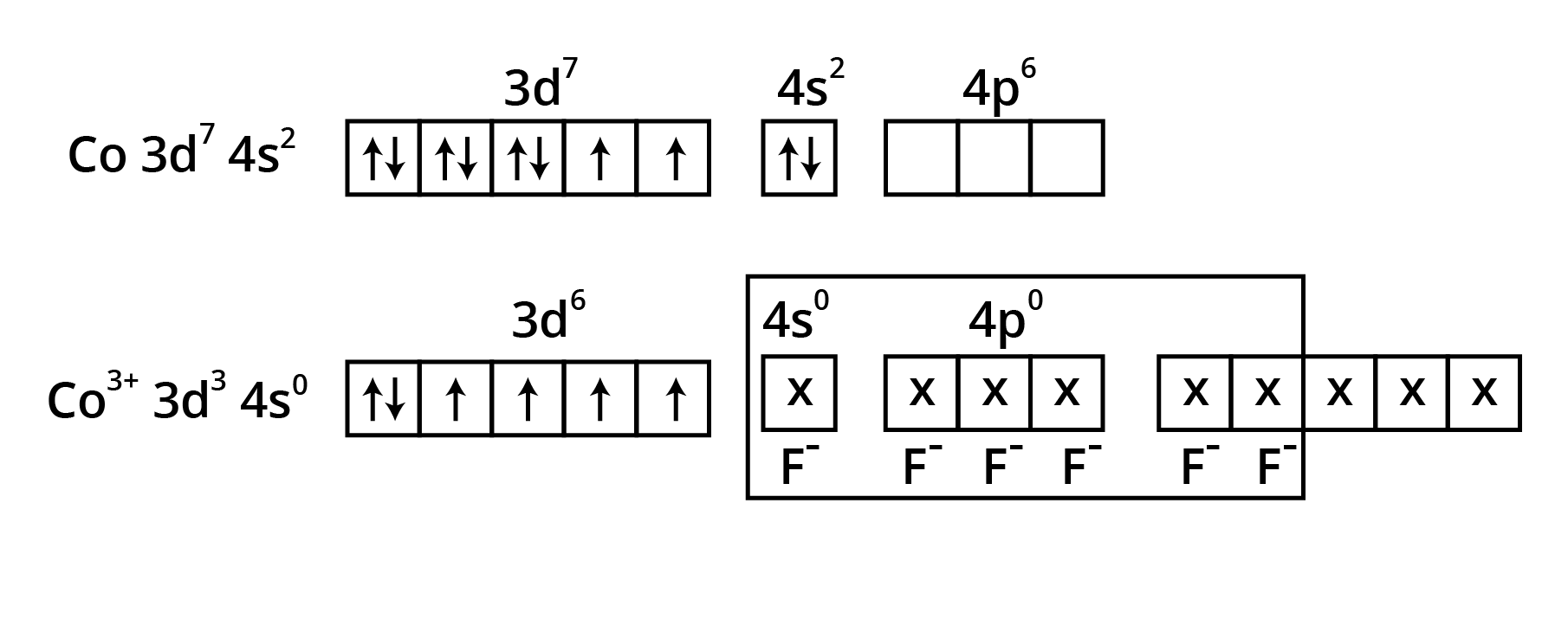
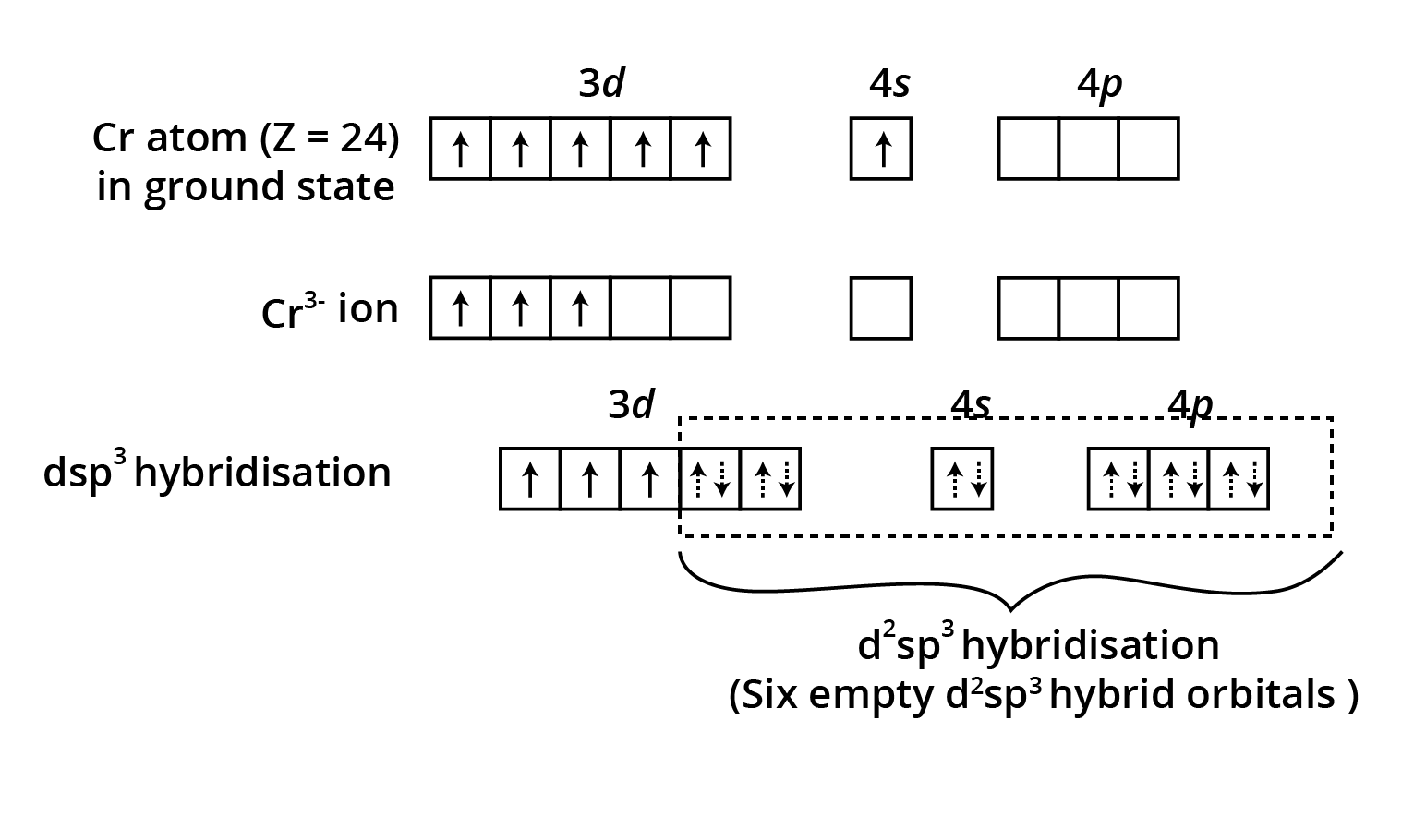
![$\mathbf{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}}$ is an inner orbital complex whereas $\mathbf{\left[\mathrm{FeF}_{6}\right]^{3-}}$ is an outer orbital complex](https://www.vedantu.com/seo/content-images/a3dfebb6-e370-416e-a60f-8726152bcd49.png)
![$\mathbf{\left[\mathrm{Fe}(\mathrm{CN})_{6}\right]^{3-}}$ is an inner orbital complex whereas $\mathbf{\left[\mathrm{FeF}_{6}\right]^{3-}}$ is an outer orbital complex](https://www.vedantu.com/seo/content-images/7107ec25-8b3b-4150-867d-58be84860fc3.png)
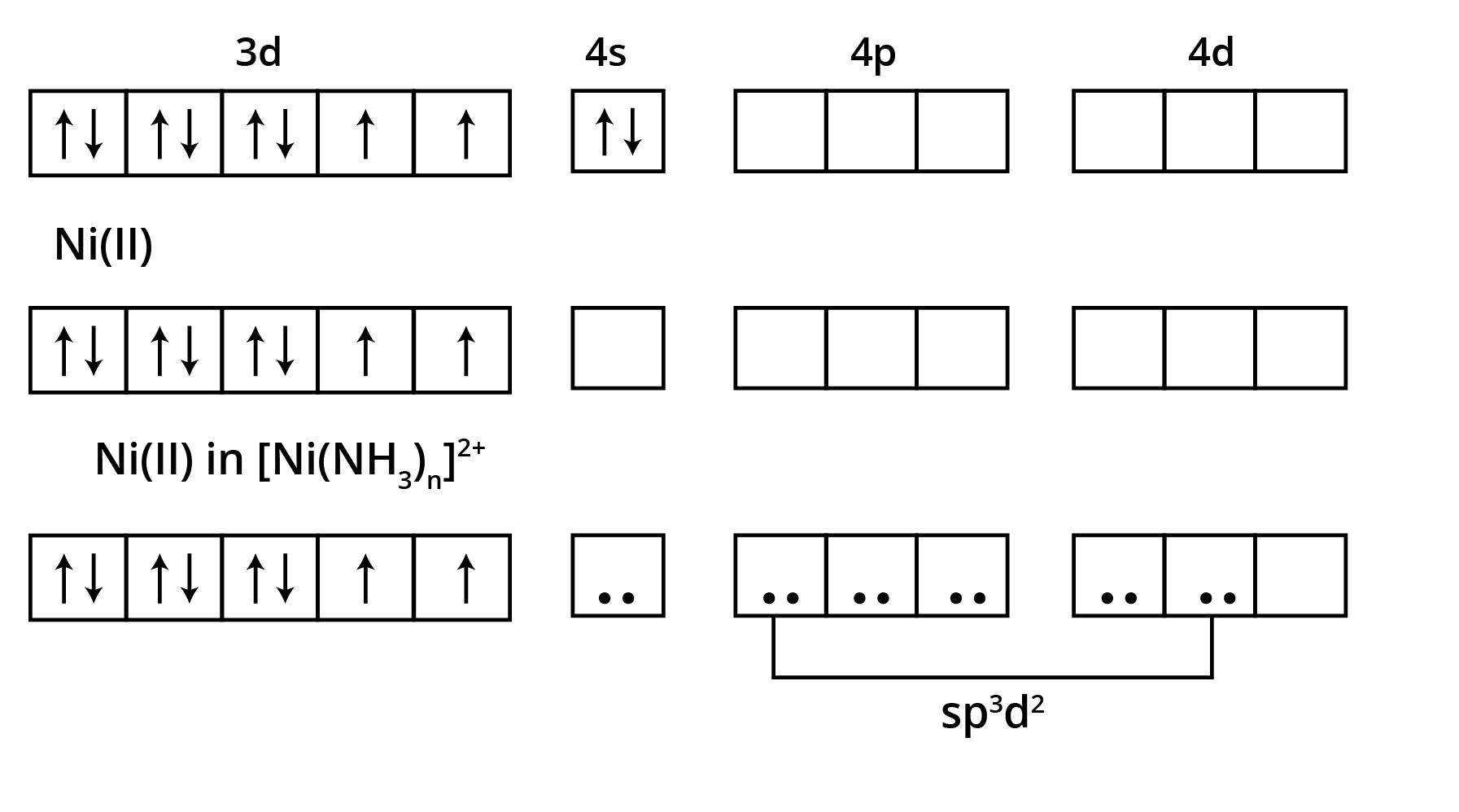
![Valance Bond Theory that diamagnetic [Ni(CN)4 ]2- has square planar structure and paramagnetic [Ni(CN)4 ]2- ion](https://www.vedantu.com/seo/content-images/18f1b4f2-8cda-4131-87d7-12967f702925.png)
![Valance Bond Theory that diamagnetic [Ni(CN)4 ]2- has square planar structure and paramagnetic [Ni(CN)4 ]2- ion](https://www.vedantu.com/seo/content-images/e0035fae-bfc1-4a0b-a155-a125eaeea97e.png)
![Two complexes of nickel [Ni(CN)4 ]2- and Ni(CO)4 have different structures but do not differ in their magnetic behaviours](https://www.vedantu.com/seo/content-images/2533e378-2c1f-447a-bf6c-fda1b64a616c.png)
![Two complexes of nickel [Ni(CN)4 ]2- and Ni(CO)4 have different structures but do not differ in their magnetic behaviours](https://www.vedantu.com/seo/content-images/f7a9035d-1c2b-4745-b1de-769c6a8feb73.png)
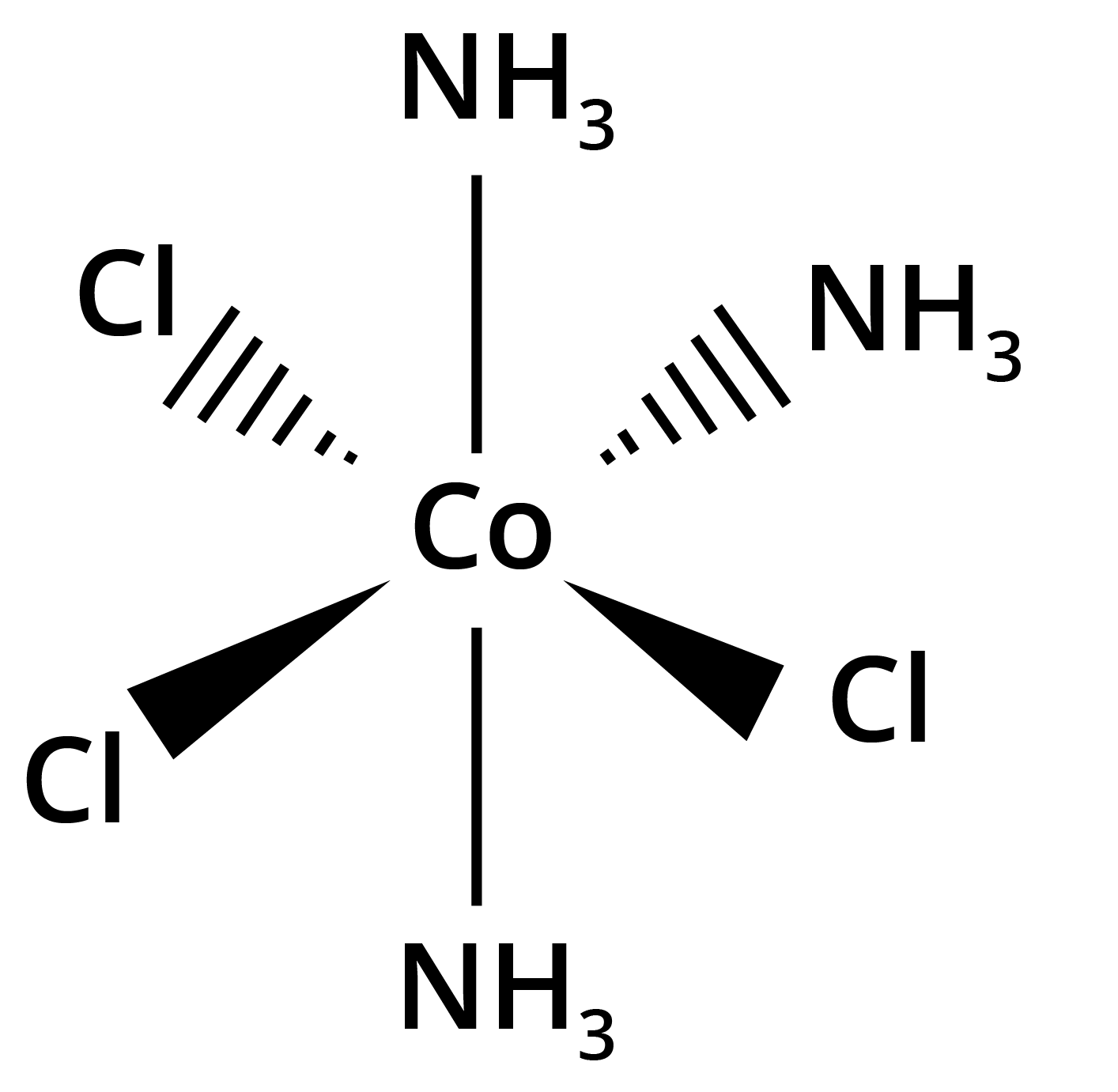
![structures of geometrical isomers of the coordination complexes– [Co(NH3 )3 Cl3 ]and [CoCl2 (en)2 ]+](https://www.vedantu.com/seo/content-images/b30fc7b3-60b3-44fd-a6af-94dcfbadef6e.png)
![The geometrical isomers of [Co(NH3 )3 Cl3 ]](https://www.vedantu.com/seo/content-images/8fec687a-b583-4af2-a0e5-a361e72db24d.png)