




An Overview of Class 11 Chemistry Effect Of Change Of Concentration On Chemical Equilibrium Experiment
During a reversible chemical process, chemical equilibrium is the state in which there is no net change in the number of reactants or products. When a chemical reaction is reversible, the products react with the original reactants as soon as they are created. When the product concentration rises, less product is produced, and the reactant concentration rises as the product concentration falls, shifting the chemical equilibrium in favour of the reactant. A bottle of effervescent refreshing drink is one example of chemical equilibrium. Carbon dioxide has been dissolved in the liquid within the container. There is also carbon dioxide gas in the area between the liquid and the lid.
Table of Content
Aim
Apparatus Required
Theory
Procedure
Observation
Result
Precautions
Lab Manual Questions
Viva Questions
Practical Based Questions
Aim
To study the change in equilibrium by varying the concentration of [Co(H2O)6]2+ and Cl- ions in their reaction.
Materials Required
Test tube
Test tube stand
Glass rod
Beakers
Conical flask
Burettes
Conc. HCl
Cobalt chloride
Acetone
Theory
According to Le Chatelier's principle, if a dynamic equilibrium is upset by changing the conditions, the equilibrium position will move to compensate for the change and restore the equilibrium.
The displacement reaction between [Co(H2O)6]2+ and Cl- take place as follows:
[Co(H2O)6]2++4Cl-→[CoCl4]2- + 6H2O
The equilibrium constant for the above reaction can be written as:
\[K = \dfrac{\left [ CoCl_4 \right ]^{2-}}{\left [ Co\left ( H_2O \right )^{2+} \right ]\left [ C^{-} \right ]^4}\]
Now, if at equilibrium, the concentration of either the [Co(H2O)6]2+ ion or the Cl- ions are raised, this would result in a rise in the concentration of the [CoCl4]2-ion and retain the value of K as constant. To put it another way, we can argue that when equilibrium shifts forward, the colour will alter to reflect this.
Procedure
Dissolve 60 ml of acetone in a 100 ml conical flask to make a blue solution, and add 0.6000 g of CoCl2.
Mark five identical test tubes with the letters A, B, C, D and E.
3.0 mL of cobalt chloride solution should be added to each from “A” to “E” accordingly, as test tubes.
Add 1.0 mL now 0.8 mL, 0.6 mL, 0.4 mL and 0.2 mL, respectively, of acetone in these syringes.
Add 0.2, 0.4, 0.6 and 0.8 ml of test tubes B, C, D and E, respectively, with water, so that combined, there is 4.0 mL of solution in each test tube.
As the amount of water is increased, observe how the mixture gradually turns from blue to pink.
Add 5 mL of distilled water to the previously made 10 mL cobalt chloride solution in acetone. You'll get a pink-coloured solution.
1.5 mL of the pink solution from above should be divided among the five test tubes marked 1, 2, 3, 4 and 5.
To make the total amount of solution in the test tubes 4 mL, add 3.0 mL, 3.5 mL, 4.0 mL, 4.5 mL and 5 mL of water to the test tubes labelled from 1 to 5 and 1mL, 1.5 mL, 2 mL, 2.5 mL and 3 mL of concentrated HCl to the test tubes 1 to 5.
Take note of how the pink solution gradually turns bright blue as the hydrochloric acid concentration rises.
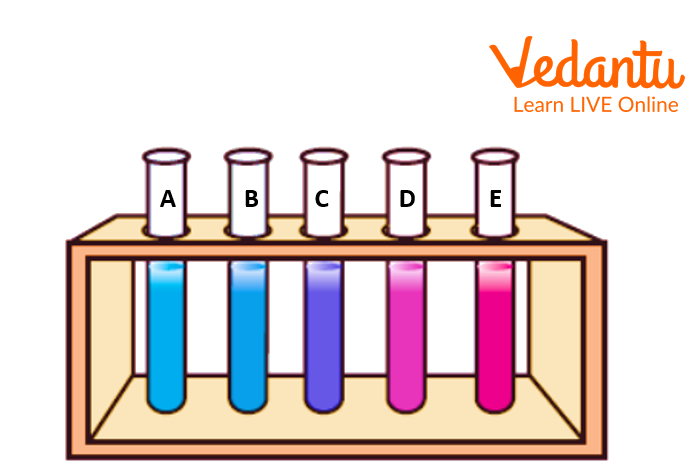
Five identical test tubes with the letters A, B, C, D and E
Observation
The Shift in Equilibrium - Addition of Water
The Shift in Equilibrium - Addition of Cl- Ion
Result
In the equilibrium reaction between [Co(H2O)6]2+ and Cl-, the equilibrium will shift to the left side (backwards) by increasing the concentration of water (product). And equilibrium will shift to the right side (forward) by increasing the concentration of Cl- ions (reactant).
Precautions
Take note of the colour shift under diffused sunlight to accurately assess the solution's colour change.
Use identical-sized boiling tubes.
Pour distilled water into the test tube.
For aeration or solution addition, use a graduated pipette or burette.
Lab Manual Questions
1. What is the purpose of using a glass rod to transfer solutions?
Ans: To avoid spills, solutions are poured using glass rods. To prevent the solution from spilling past the lip of a beaker and into the collecting vessel, a glass rod is pushed up against the beaker's pouring edge.
2. Why does acetone keep close after usage?
Ans: Acetone and alcohol are inflammable, do not let the bottles open when not in use. Keep the bottles away from flames.
3. Why were identical-diameter test tubes used in this experiment?
Ans: To measure the introduced solutions precisely to conduct comparison research.
4. What alters equilibrium when HCl is added?
Ans: When HCl is added, chloride ion concentration increases dramatically, upsetting the equilibrium.
Viva Questions
1. Define the law of mass action.
Ans: The law of mass action also says that at a condition of chemical equilibrium, the ratio of reactant concentration to product concentration is constant.
2. Define reversible chemical reactions.
Ans: The simultaneous transformation of reactants into products and products back into reactants is known as a reversible reaction.
3. What is meant by the dynamic nature of equilibrium?
Ans: When a system is in a state of dynamic equilibrium, the reversible reaction that is occurring in it no longer changes the ratio of reactants to products, but there is still the movement of substances between the reactants and products.
4. Define the law of chemical equilibrium.
Ans: The product of the molar concentration of the products divided by the product of the molar concentrations of the reactants, each concentration increased to the power corresponding to its coefficients, is constant at a given temperature for a reversible reaction in equilibrium. The name of this constant is the equilibrium constant.
5. What happens at the increasing temperature of an equilibrium system?
Ans: The equilibrium constant's value falls as the temperature rises. A rise in temperature increases the value of the equilibrium constant when the forward reaction is endothermic. As the temperature fluctuates, so does the equilibrium position.
6. What is meant by chemical equilibrium?
Ans: Chemical equilibrium occurs in a chemical reaction when the rate of the forward reaction equals the rate of the backward reaction.
7. What does K indicate in an equilibrium reaction?
Ans: The value of K represents the products-to-reactants ratio at equilibrium.
8. What is KC in an equilibrium reaction?
Ans: KC stands for Equilibrium constant measured in moles per litre.
9. What is the colour of [Co(H2O)6]2+ ions?
Ans: Pink
10. What is the colour of [CoCl4]2- ion?
Ans: Blue
Practical Based Questions
If the concentration of any one of the reactants in a reversible chemical process doubles while it is in equilibrium, the equilibrium constant will ____.
Be doubled
Become one-fourth
Be halved
Remain the same
Ans: If the concentration of any one of the reactants in a reversible chemical process doubles while it is in equilibrium, the equilibrium constant will remain the same.
The rate of forwarding to the reverse reaction if a system is in equilibrium is?
Less
High
Equal
None
Ans: If a system is at equilibrium, the rate of forward-to-reverse reaction is equal.
What happens if an equilibrium is added while maintaining the same volume of an inert gas?
Less product will form
More products will form
More reactants will form
Equilibrium will remain unchanged
Ans: If equilibrium is added while maintaining the same volume of inert gas, equilibrium will remain unchanged.
What happens to equilibrium on doubling P and V at constant T?
Become double
Remain constant
Become one-fourth
None
Ans: The equilibrium remains constant.
What happens if the temperature of the chemical equilibrium reaction mixture is increased?
Equilibrium remains constant
Equilibrium shifts towards an exothermic direction
Equilibrium shifts towards an endothermic direction
None of the three options
Ans: The equilibrium shifts towards the endothermic direction if the temperature rises.
What is the role of distilled water in laboratories?
Indicator
Solvent
Universal solvent
None
Ans: Distilled water is used as a universal solvent.
Choose the correct statement about chemical equilibrium.
It is not affected by pressure
It is affected by the catalyst
It is affected by P, T and V
None of these
Ans: The chemical equilibrium is affected by pressure, volume, and temperature.
The colour of [Co(H2O)6]2+ ion is _____.
Blue
Black
Pink
Yellow
Ans: The colour of [Co(H2O)6]2+ ion is Pink.
Select the wrong statement.
The colour of [CoCl4]2- ion is black.
P, V and T affect the chemical equilibrium.
Equilibrium can shift on change in concentration.
None of these
Ans: The colour of [CoCl4]2- ion is blue.
Which of the following statements is true?
The chemical equilibrium is affected by the catalyst.
The chemical equilibrium is not affected by the catalyst.
A catalyst does not speed up the reaction.
None of these
Ans: The chemical equilibrium is not affected by the catalyst.
Conclusion
From the above experiment, we can conclude that a change in the concentration of substances can alter the equilibrium. Here, the increase in the concentration of water shifts the equilibrium to the left and increases in the concentration of chloride ions shift the equilibrium to the right. And this shift is due to Le Chatelier's principle, if a dynamic equilibrium is upset by changing the conditions, the equilibrium position will move to compensate for the change and restore the equilibrium.
FAQs on Class 11 Chemistry Effect Of Change Of Concentration On Chemical Equilibrium Experiment
1. What is the effect of changing the concentration of a substance on a chemical equilibrium as per Le Chatelier's principle?
According to Le Chatelier's principle, if the concentration of any reactant or product in a reaction at equilibrium is changed, the system will shift in a direction that counteracts this change. For instance, increasing the concentration of a reactant will shift the equilibrium to the right (forward direction) to consume the added reactant. Conversely, increasing the concentration of a product will shift it to the left (reverse direction).
2. For the reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), what are the expected shifts in equilibrium for the following changes, which are frequently asked in exams?
For this important industrial reaction (Haber's process), the equilibrium shifts as follows to minimise the effect of the change:
- Adding N₂ or H₂: Increasing the concentration of reactants will favour the forward reaction, causing the equilibrium to shift to the right to produce more NH₃.
- Removing NH₃: Decreasing the concentration of the product will also favour the forward reaction, causing the equilibrium to shift to the right to replenish the NH₃.
- Adding NH₃: Increasing the product concentration will favour the reverse reaction, causing the equilibrium to shift to the left to decompose NH₃ into N₂ and H₂.
3. How does a change in pressure affect a gaseous chemical equilibrium? Explain with a relevant example.
A change in pressure affects only those equilibria that involve gaseous components and where the total number of moles of gaseous reactants is different from the total number of moles of gaseous products. An increase in pressure will shift the equilibrium towards the side with fewer moles of gas. For example, in the reaction N₂(g) + 3H₂(g) ⇌ 2NH₃(g), there are 4 moles of gas on the reactant side and 2 moles on the product side. Therefore, an increase in pressure favours the forward reaction (production of ammonia).
4. Why is chemical equilibrium considered to be “dynamic in nature”? Justify with an example.
Chemical equilibrium is described as dynamic in nature because, at the point of equilibrium, the reaction has not stopped. Instead, the rate of the forward reaction becomes equal to the rate of the reverse reaction. Macroscopically, properties like concentration, pressure, and colour appear constant. However, at a molecular level, reactant molecules are continuously converting into products, and product molecules are converting back into reactants at the same speed, resulting in no net change in the concentrations of the species involved.
5. What is the role of a catalyst in a reversible reaction, and does it change the equilibrium position?
A catalyst increases the rate of a chemical reaction by providing an alternative pathway with lower activation energy. In a reversible reaction, a catalyst increases the rate of both the forward and reverse reactions to the same extent. Consequently, a catalyst helps the reaction to attain equilibrium more quickly, but it does not alter the equilibrium constant (Kc) or the final concentrations of reactants and products at equilibrium.
6. How can the reaction quotient (Qc) be used to predict the direction of a reaction to attain equilibrium?
The reaction quotient (Qc) is a crucial value that helps predict the direction in which a reversible reaction will shift to reach equilibrium. It is calculated in the same way as the equilibrium constant (Kc) but using concentrations at any given moment, not just at equilibrium. The direction is determined by comparing Qc with Kc:
- If Qc < Kc, the ratio of products to reactants is too small. The reaction will proceed in the forward direction to reach equilibrium.
- If Qc > Kc, the ratio of products to reactants is too large. The reaction will proceed in the reverse direction.
- If Qc = Kc, the system is already at equilibrium, and there will be no net reaction.
7. What is the effect of adding an inert gas on an equilibrium mixture at (a) constant volume and (b) constant pressure?
This is a key conceptual question often asked to test a student's understanding of Le Chatelier's principle:
- (a) At Constant Volume: Adding an inert gas at constant volume increases the total pressure of the system. However, the partial pressures or concentrations of the reacting species do not change. Therefore, adding an inert gas at constant volume has no effect on the equilibrium.
- (b) At Constant Pressure: Adding an inert gas at constant pressure increases the total volume. This causes the partial pressures and concentrations of all reacting species to decrease. The equilibrium will then shift in the direction that leads to an increase in the number of moles of gas to counteract the volume increase.
























