Science Notes for Chapter 4 Structure of the Atom Class 9 - FREE PDF Download
CBSE Notes Class 9 Science Chapter 4 - Structure of the Atom - 2025-26
FAQs on CBSE Notes Class 9 Science Chapter 4 - Structure of the Atom - 2025-26
1. What are the main concepts summarised in Class 9 Science Chapter 4 Structure of the Atom revision notes?
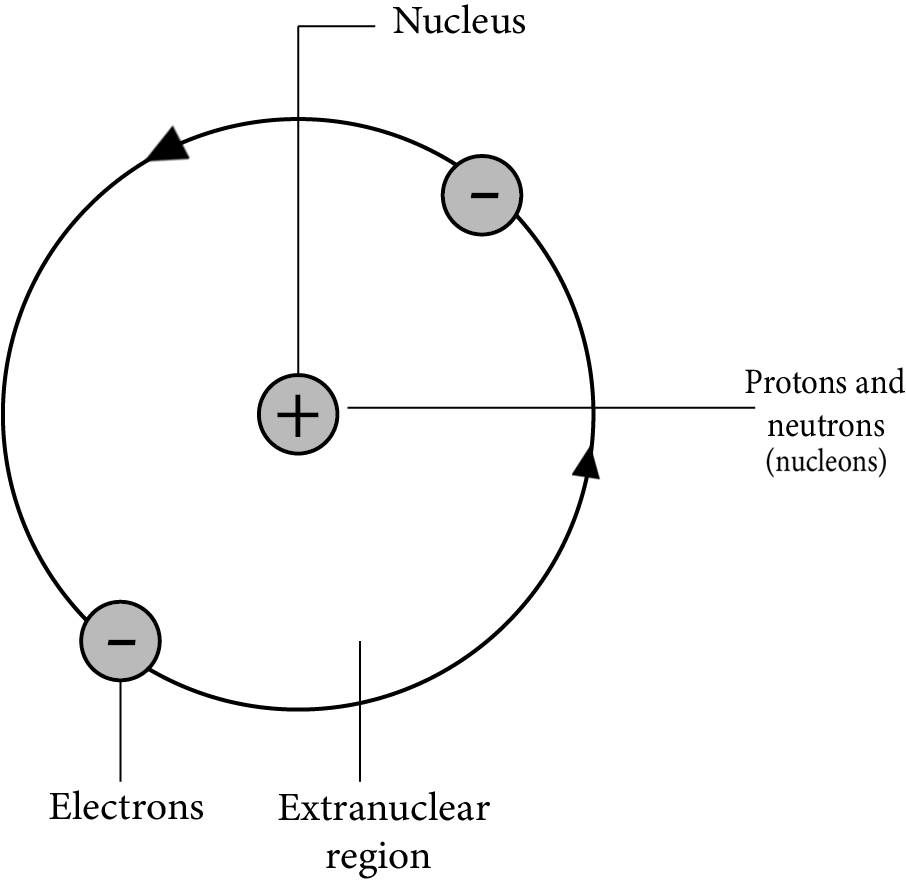
The revision notes for Structure of the Atom in Class 9 Science focus on the discovery of subatomic particles (electrons, protons, neutrons), key atomic models (Thomson, Rutherford, Bohr), electron configuration, atomic and mass numbers, and isotopes and isobars. Together, these concepts build a foundation for understanding how atoms are structured and behave.
2. How is electron arrangement explained for the first 18 elements in the revision notes?
Electron arrangement in the first 18 elements is described using Bohr and Bury's rules. Electrons fill shells in a stepwise sequence, with a maximum of 2 electrons in the K-shell, up to 8 in the L-shell, and so on. Each element's valency and properties are linked directly to its outermost shell's electron count, helping students understand periodic trends.
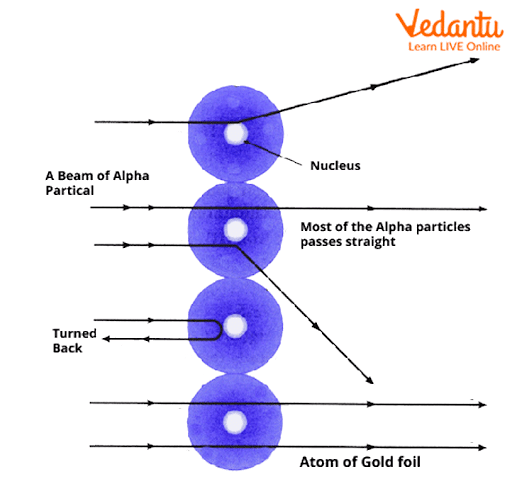
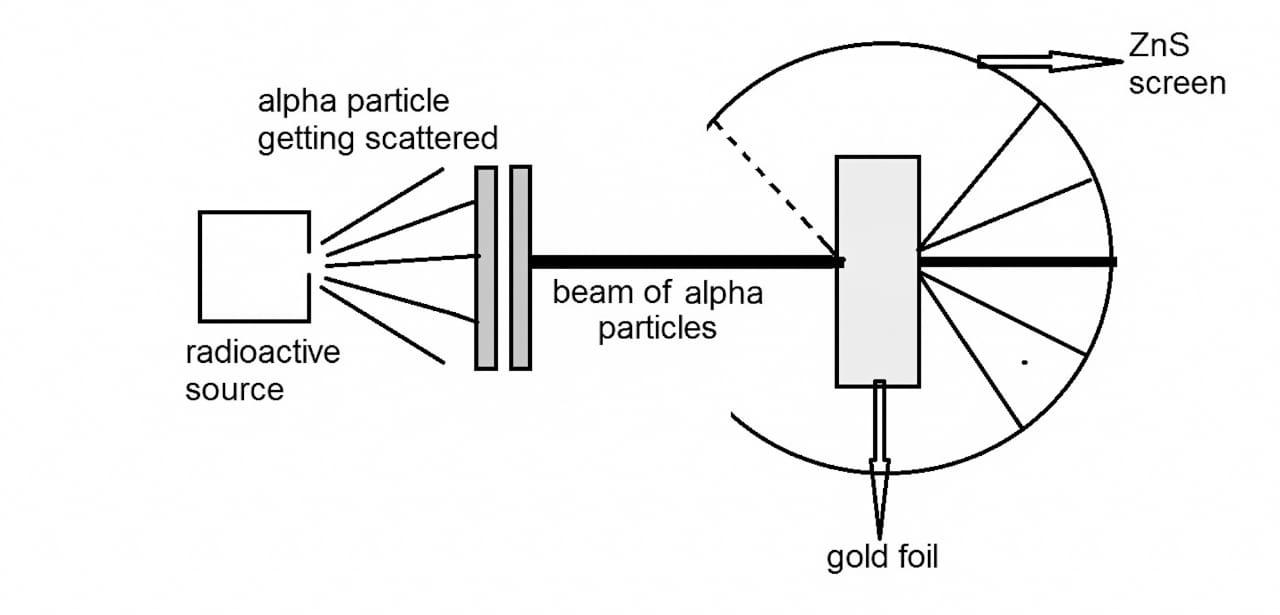
3. Why did Rutherford's model of the atom require improvement, as highlighted in these notes?
Rutherford's model proposed a dense, positively charged nucleus with electrons orbiting it, but it couldn't explain atomic stability. According to classical physics, orbiting electrons would lose energy and collapse into the nucleus. The notes explain that Bohr's model addressed this by introducing fixed energy levels for electrons.
4. What is the importance of understanding isotopes and isobars in atomic structure revision?
Isotopes and isobars are crucial for understanding atomic mass variation and element identification. Isotopes have the same atomic number but different mass numbers due to varying neutrons, while isobars have the same mass number but different atomic numbers. Recognising these helps in topics like radioactivity, chemical properties, and physical properties of elements.
5. How do the revision notes structure the key topics to support efficient exam revision?
The notes use a logical flow: starting with subatomic particle discoveries, explaining each atomic model's contributions, detailing electron configuration, and concluding with special cases like isotopes and isobars. This step-by-step approach helps students retain and connect concepts easily during quick revision.
6. What strategies do the notes suggest for effectively revising atomic structure concepts?
The revision notes recommend strategies such as
- reviewing diagrams of atomic models,
- summarising each section in your own words,
- using visual aids for electron arrangements,
- testing knowledge with practice questions,
- and regular, scheduled revision to reinforce understanding.
7. Why is it essential to understand the difference between atomic number and mass number when revising this chapter?
Understanding the atomic number (number of protons) and mass number (sum of protons and neutrons) is essential as they define an element's identity and properties. The distinction helps in calculating isotopes, representing elements symbolically, and interpreting periodic table trends.
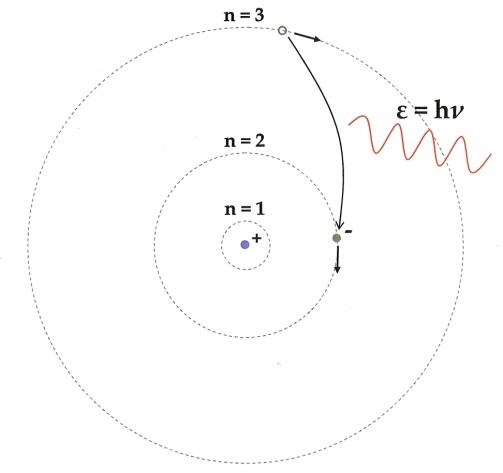
8. What role does the Bohr model play in forming the basis of modern atomic theory, as per the notes?
The Bohr model introduced the idea of quantized electron orbits where electrons revolve in specific energy levels without radiating energy. This theory explained atomic stability and the discrete line spectra in elements, serving as a bridge to modern quantum mechanics.
9. How can concise revision notes help students prepare for board exams in Science?
Concise revision notes summarise critical information, making it easy to revise important points quickly before exams. They provide clear explanations, diagrams, and key terms, helping students to remember essential facts and solve application-based questions confidently.
10. What misconception about atomic structure is corrected through revision notes on this chapter?
A common misconception is that electrons revolve around the nucleus like planets forever, which the notes clarify as inaccurate according to classical physics. The Bohr model corrects this by stating electrons only occupy stable, quantized orbits, helping students avoid confusion and future errors in understanding atomic stability.




















 Watch Video
Watch Video