How to Write Full Mark Answers for Acidic, Basic, and Neutral Substances?
FAQs on NCERT Solutions For Class 7 Science Chapter 2 Exploring Substances Acidic, Basic, And Neutral - 2025-26
1. What are acids, bases and neutrals in Class 7 Science?
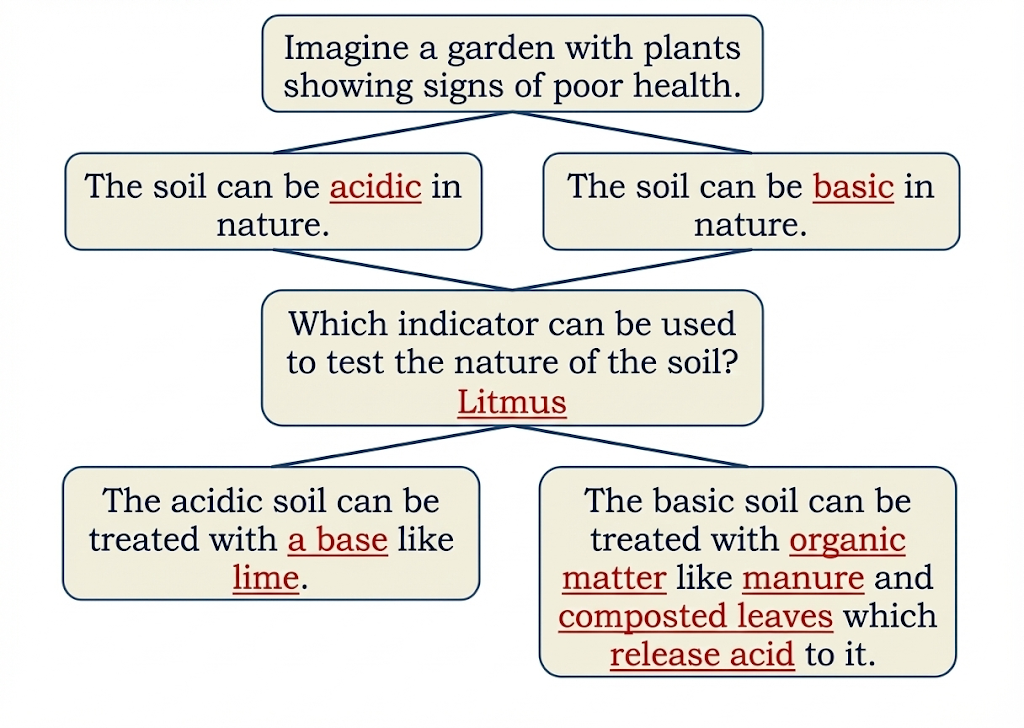
Acids, bases, and neutrals are three main categories of substances discussed in Class 7 Science Chapter 2.
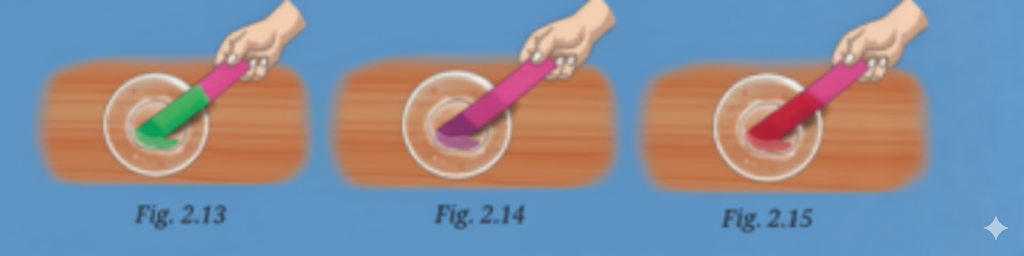
- Acids: Substances that taste sour and turn blue litmus red (e.g., lemon juice, vinegar).
- Bases: Substances that feel soapy and turn red litmus blue (e.g., soap solution, baking soda).
- Neutral Substances: Substances that do not affect litmus paper (e.g., water, salt solution).
- Indicators like litmus help identify whether a substance is acidic, basic, or neutral.
- This concept is essential for understanding chemical reactions, everyday applications, and exam questions in the CBSE Class 7 syllabus.
2. How to prepare for Class 7 Science Chapter 2?
To prepare well for Class 7 Science Chapter 2: Exploring Substances: Acidic, Basic, and Neutral, follow these steps:
- Read the NCERT textbook thoroughly and highlight key definitions (acid, base, neutral, indicators).
- Practice stepwise NCERT Solutions and back exercise questions.
- Study diagrams showing litmus test procedures and label them properly.
- Revise with flash notes and important formulae lists.
- Attempt sample papers and previous year questions.
- Download the free, CBSE-aligned solutions PDF for revision.
- Focus on common marking schemes and use keywords in your answers.
3. Which are the important questions of Chapter 2 Science Class 7?
Important exam questions from Chapter 2 often include:
- Definitions of acids, bases, neutrals, and indicators.
- Differences between acids and bases with examples.
- Experiments using litmus paper or turmeric as an indicator.
- Diagram-based questions (e.g., identifying substances using indicators).
- MCQs or reasoning about everyday acidic/basic items.
- Practising exercise and intext questions from NCERT is vital to score full marks in CBSE exams.
4. How to write stepwise NCERT answers to score full marks?
Writing stepwise NCERT answers for Class 7 Science maximises marks in CBSE exams:
- Start with a clear definition or concept statement.
- Use bullets or steps for explanations or processes.
- Include examples for clarity.
- Label diagrams wherever required and refer to them in your answer.
- Highlight important terms and keywords from the question.
- Keep answers structured: introduction, main body (steps), and conclusion.
- Follow CBSE marking scheme and answer length guidelines.
5. Are diagrams or definitions mandatory in answers?
In CBSE Class 7 Science exams, diagrams and definitions are often required for full marks:
- Definitions of terms like acid, base, and indicator are essential when asked directly.
- Diagrams (e.g., showing a litmus paper test) are compulsory if the question mentions ‘draw’ or ‘explain with diagram’.
- Properly labelled diagrams and accurate definitions help score stepwise marks and reduce chances of missing out on key points.
- Always check the question instructions and CBSE marking scheme for requirements.
6. Where can I download the chapter’s solutions PDF?
Students can download the free PDF of NCERT Solutions for Class 7 Science Chapter 2 from leading educational platforms that offer CBSE content:
- Search for “Class 7 Science Chapter 2 Solutions PDF download”.
- Look for CBSE syllabus-aligned, teacher-reviewed resources.
- Ensure the PDF includes stepwise answers, diagrams, and exam-keyword focus.
- Use the downloaded PDF offline for revision, practice, and last-minute exam prep.
7. Do examiners award partial marks for correct steps even if the final answer is wrong?
Yes, in CBSE exams, examiners usually award partial marks for correct steps:
- Step marking is used for long and multi-step questions in Class 7 Science.
- If you write some correct steps but make a calculation or conclusion error, you can still earn marks for the correct procedure.
- Always show all steps clearly and use scientific terms and definitions.
- Following the NCERT Solutions stepwise method increases your chances of earning maximum possible marks.
8. What are the most important topics from this chapter?
The most important topics from Class 7 Science Chapter 2: Exploring Substances: Acidic, Basic, and Neutral are:
- Definitions and examples of acids, bases, and neutral substances.
- Acid-base indicators (litmus, turmeric, china rose) and their uses.
- Neutralisation reactions and their everyday relevance.
- Experiment-based questions involving household items.
- Diagram labelling and stepwise explanations.
- Understanding how to use and interpret indicators is key for exams.
9. How to learn diagrams/maps for this chapter?
To master diagrams for Class 7 Science Chapter 2:
- Practice drawing key experiment setups (e.g., litmus test apparatus).
- Follow CBSE conventions: draw neatly, use a pencil, and label every part clearly.
- Study diagram labelling tips in NCERT Solutions and exam guides.
- Revise using flashcards or practice sheets focused on commonly asked diagrams.
- Refer to NCERT textbook and solution images for correct format.
- Diagrams should be simple, accurate, and labelled to match answer keywords.
10. Are NCERT Solutions enough for Class 7 Science exams?
NCERT Solutions provide a strong foundation for scoring well in Class 7 Science exams:
- They cover all NCERT exercise and intext questions aligned with CBSE marking schemes.
- Stepwise explanations, diagrams, and key definitions help secure full marks.
- For advanced preparation, also practice exemplar questions and MCQs.
- Use revision notes and sample papers to test understanding and speed.
- NCERT Solutions are essential for everyday study and final exam revision.