
Total number of lone pair electrons in ${{I}_{3}}^{-}$ ion is:
(A) 9
(B) 12
(C) 3
(D) 6
Answer
233.1k+ views
Hint: Before finding the total number of lone pairs, we first need to be able to find if the given ion is ionic or covalent in nature and then we need to see the electronic configuration of the atoms involved. Only then we shall be able to determine the exact number of bonded pairs and lone pairs of electrons.
Complete step by step solution:
-VSEPR theory gives us the information not only of the hybridization and the geometry of the molecule but it also tells us about the exact number of bond pairs and the lone pairs.
-Every atom has a certain number of electrons in their outermost shell which we call the valence electrons and the atoms try to complete their octet by filling their shells. They can do it either by sharing the electrons or by losing/gaining the electrons.
-Electrons are gained/lost when there is a good amount of electronegativity difference between the atoms forming the molecules else they share electrons.
-In the ion given, we see that there is only one type of atom present and its atomic number is 53. So its electronic configuration can be represented as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}4{{p}^{6}}5{{s}^{2}}4{{d}^{10}}5{{p}^{5}}$
-If we see the ion given, we see that the atom iodine belongs to is group 7 and so it has 7 electrons in its outermost shell which is also visible from its electronic configuration. Now to complete its octet rule, it has to gain 1 electron only.
-Keeping one I atom at the centre and other 2 bonded to it from left and right sides, we find that the central atom will possess a total 9 electrons now, 7 of its own and 2 from sharing with the adjacent atoms. So, a negative sign appears on that atom to validate octet rule.
-The adjacent I atoms only needed one more electron which they will get by bonding with the centre I atom. Thus there are 2 bonded electrons and rest are lone pair electrons. There were 7 electrons in the adjacent atom, 1 of which takes part in bonding. So now there will remain 6 electrons, thus, 3 electron lone pairs each from the adjacent atom.
-The central atom has 7 electrons and gained 2 electrons from the adjacent atoms and so it has a total 9 electrons and therefore a negative sign. 2 electrons take part in bonding and so it is also left with 6 electrons which means 3 lone pairs.
-Thus we can see that the total number of lone pairs in the whole ion will be 3+3+3=9.
Therefore the correct answer is option (A) 9.
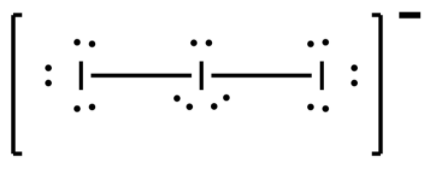
Note: Keep in mind that the shape of the ion can either be linear or bent, both are possible. Both the structures will be the same except for their packing efficiency which will change their melting and boiling point by a slight amount. Their shape comes out as such to keep the lone pairs away from each other as they repel each other. So they are at equatorial positions while atoms at axial positions.
Complete step by step solution:
-VSEPR theory gives us the information not only of the hybridization and the geometry of the molecule but it also tells us about the exact number of bond pairs and the lone pairs.
-Every atom has a certain number of electrons in their outermost shell which we call the valence electrons and the atoms try to complete their octet by filling their shells. They can do it either by sharing the electrons or by losing/gaining the electrons.
-Electrons are gained/lost when there is a good amount of electronegativity difference between the atoms forming the molecules else they share electrons.
-In the ion given, we see that there is only one type of atom present and its atomic number is 53. So its electronic configuration can be represented as $1{{s}^{2}}2{{s}^{2}}2{{p}^{6}}3{{s}^{2}}3{{p}^{6}}4{{s}^{2}}3{{d}^{10}}4{{p}^{6}}5{{s}^{2}}4{{d}^{10}}5{{p}^{5}}$
-If we see the ion given, we see that the atom iodine belongs to is group 7 and so it has 7 electrons in its outermost shell which is also visible from its electronic configuration. Now to complete its octet rule, it has to gain 1 electron only.
-Keeping one I atom at the centre and other 2 bonded to it from left and right sides, we find that the central atom will possess a total 9 electrons now, 7 of its own and 2 from sharing with the adjacent atoms. So, a negative sign appears on that atom to validate octet rule.
-The adjacent I atoms only needed one more electron which they will get by bonding with the centre I atom. Thus there are 2 bonded electrons and rest are lone pair electrons. There were 7 electrons in the adjacent atom, 1 of which takes part in bonding. So now there will remain 6 electrons, thus, 3 electron lone pairs each from the adjacent atom.
-The central atom has 7 electrons and gained 2 electrons from the adjacent atoms and so it has a total 9 electrons and therefore a negative sign. 2 electrons take part in bonding and so it is also left with 6 electrons which means 3 lone pairs.
-Thus we can see that the total number of lone pairs in the whole ion will be 3+3+3=9.
Therefore the correct answer is option (A) 9.

Note: Keep in mind that the shape of the ion can either be linear or bent, both are possible. Both the structures will be the same except for their packing efficiency which will change their melting and boiling point by a slight amount. Their shape comes out as such to keep the lone pairs away from each other as they repel each other. So they are at equatorial positions while atoms at axial positions.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




