
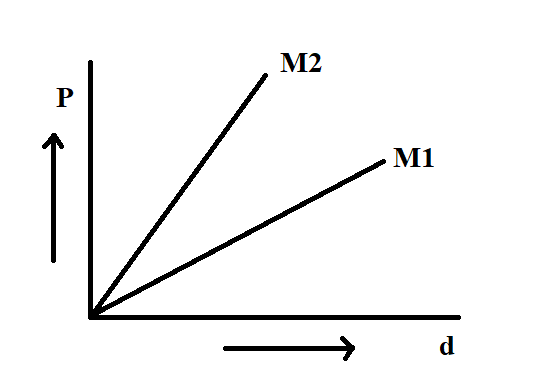
In the Boyle's law experiment, the graph drawn between pressure and density for a given temperature of different gases are the straight lines then the molecular weights have the relation
A) ${M_1} > {M_2}$
B) ${M_2} > {M_1}$
C) ${M_1} = {M_2}$
D) $M_2^2 = {M_1}$
Answer
233.1k+ views
Hint: The graph is between pressure and density. To find the relation between the two masses can be obtained by using the expression for density and Boyle's law for an ideal gas. The density of a gas is the ratio of its mass to the volume. Boyle's law graphs are plotted with pressure on one axis and reciprocal of volume on the other axis. In this graph mass of the gas is multiplied by the volume to get density on the x-axis.
Formula used:
$d = \dfrac{m}{V}$ (Where d stands for the density, m stands for the given mass and V stands for the volume)
The ideal gas equation is given by,
$PV = nRT$ (where P is the pressure of the gas, V is the volume of the gas, n stands for the number of moles, R stands for the universal gas constant and T is the temperature of the gas)
Complete step by step solution:
We know that in the graph, pressure versus density is shown,
We know the expression for density,
Density $d = $$\dfrac{{mass}}{{volume}} = \dfrac{m}{V}$
Boyle's law for ideal gases is given by,
$PV = nRT$
$n$ is the number of moles which is given by, $\dfrac{{mass(given)}}{{Mass(molecular)}} = \dfrac{m}{M}$
where$ M$ is the molecular weight.
Substituting the value of $n$ in the ideal gas equation,
$PV = \dfrac{m}{M}RT$
Rearranging this equation, we get the density
Density, $(d)= \dfrac{m}{V} = \dfrac{{PM}}{{RT}}$
The graph of pressure versus density is shown in the figure as a straight line.
From the equation, we get that the slope of this line is, $\dfrac{{RT}}{M}$
As the molecular mass is in the denominator, the line with a greater slope will be having less molecular mass.
Since ${M_2}$ is steeper than ${M_1}$, ${M_1}$ will be greater than ${M_2}$.
Hence, the answer is (A), ${M_1} > {M_2}$.
Note: The slope will be more for a steeper line. Boyle’s law states that for a constant temperature the volume of a given mass of a gas is inversely proportional to volume. Gases are liquefied by using the principle of Boyle’s law. Boyle's law has many real-life applications also. The injection syringe is one example of Boyle's law.
Formula used:
$d = \dfrac{m}{V}$ (Where d stands for the density, m stands for the given mass and V stands for the volume)
The ideal gas equation is given by,
$PV = nRT$ (where P is the pressure of the gas, V is the volume of the gas, n stands for the number of moles, R stands for the universal gas constant and T is the temperature of the gas)
Complete step by step solution:
We know that in the graph, pressure versus density is shown,
We know the expression for density,
Density $d = $$\dfrac{{mass}}{{volume}} = \dfrac{m}{V}$
Boyle's law for ideal gases is given by,
$PV = nRT$
$n$ is the number of moles which is given by, $\dfrac{{mass(given)}}{{Mass(molecular)}} = \dfrac{m}{M}$
where$ M$ is the molecular weight.
Substituting the value of $n$ in the ideal gas equation,
$PV = \dfrac{m}{M}RT$
Rearranging this equation, we get the density
Density, $(d)= \dfrac{m}{V} = \dfrac{{PM}}{{RT}}$
The graph of pressure versus density is shown in the figure as a straight line.
From the equation, we get that the slope of this line is, $\dfrac{{RT}}{M}$
As the molecular mass is in the denominator, the line with a greater slope will be having less molecular mass.
Since ${M_2}$ is steeper than ${M_1}$, ${M_1}$ will be greater than ${M_2}$.
Hence, the answer is (A), ${M_1} > {M_2}$.
Note: The slope will be more for a steeper line. Boyle’s law states that for a constant temperature the volume of a given mass of a gas is inversely proportional to volume. Gases are liquefied by using the principle of Boyle’s law. Boyle's law has many real-life applications also. The injection syringe is one example of Boyle's law.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




