Chemical Effects of Electric Current Class 8 Extra Questions and Answers Free PDF Download
FAQs on CBSE Important Questions for Class 8 Science Chemical Effects of Electric Current - 2025-26
1. What are conductors and insulators? As per the 2025-26 CBSE pattern, provide two examples for each to secure full marks in a short-answer question.
For a 2-mark question, the answer should be precise. Conductors are materials that allow electric current to pass through them easily. Examples include metals like copper and iron. Insulators are materials that do not allow electric current to pass through them. Examples include rubber and plastic.
2. List four important applications of electroplating that are frequently asked in Class 8 exams.
Four key applications of electroplating are:
Corrosion Prevention: Iron objects, like car parts and bathroom fittings, are coated with chromium or zinc to prevent rusting.
Jewellery: Less expensive metals are coated with a thin layer of gold or silver to make affordable, attractive jewellery.
Food Storage: Tin cans used for storing food are made by electroplating tin onto iron. Tin is less reactive than iron, protecting the food from spoiling.
Strengthening Objects: A layer of a stronger metal can be electroplated onto a weaker one to improve its durability and appearance.
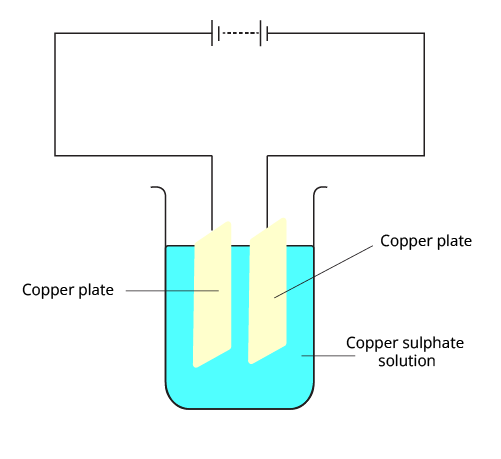
3. Explain the process of electroplating an iron spoon with copper. What are the essential components (anode, cathode, electrolyte) for this setup?
To electroplate an iron spoon with copper, a specific setup is required. The essential components are:
Anode (Positive Electrode): A plate of pure copper.
Cathode (Negative Electrode): The iron spoon to be plated.
Electrolyte: A solution of a copper salt, such as copper sulphate (CuSO₄) dissolved in water.
When an electric current is passed through the copper sulphate solution, the solution dissociates into copper ions (Cu²⁺) and sulphate ions (SO₄²⁻). The positive copper ions are attracted to the negatively charged iron spoon (cathode) and get deposited on it as a thin layer of pure copper. Simultaneously, an equal amount of copper from the copper plate (anode) dissolves into the solution to replenish the copper ions lost, ensuring the process continues.
4. What are the three main chemical effects that can be observed when electric current passes through a conducting liquid?
The three primary chemical effects observed when an electric current passes through a conducting solution (like acidified water or a salt solution) are:
Formation of Gas Bubbles: Bubbles of gas may form on the electrodes. For example, during the electrolysis of water, hydrogen and oxygen gas bubbles are produced.
Deposition of Metal: Metal from the solution can get deposited on the negative electrode (cathode), as seen in the process of electroplating.
Change in Colour of the Solution: The chemical reactions can cause the colour of the electrolyte to change, indicating that a new substance has been formed.
5. Why is pure or distilled water considered a poor conductor of electricity, while tap water conducts it effectively? This is a common HOTS question.
The ability of a liquid to conduct electricity depends on the presence of free-moving charged particles called ions. Distilled water is extremely pure and contains very few ions, making it a poor conductor. Tap water, on the other hand, contains dissolved salts and minerals from its source. These substances dissociate into positive and negative ions, which are free to move and carry electric current through the water, making it a conductor.
6. For a class activity, why is an LED often a better choice than a standard torch bulb in a conductivity tester, especially for testing weak electrolytes like lemon juice?
An LED (Light Emitting Diode) is a better choice than a standard torch bulb for a few key reasons:
High Sensitivity: An LED can light up even when a very weak electric current passes through it. Many substances, like lemon juice or vinegar, are weak conductors and only allow a small current to flow.
Low Power Requirement: A standard bulb requires a significant current to heat its filament until it glows. The weak current from a poor electrolyte is often insufficient to make the bulb glow, leading to the incorrect conclusion that the liquid is a non-conductor. An LED avoids this issue.
7. To prevent rusting, iron objects are coated with zinc in a process called galvanisation. Why is a more reactive metal like zinc preferred over a less reactive one like tin for this purpose?
Zinc is preferred over tin for protecting iron due to a principle called sacrificial protection. Zinc is more reactive than iron. If the zinc coating on a galvanised object gets scratched, the exposed zinc will corrode (react with air and water) in preference to the iron. The zinc sacrifices itself to protect the iron. In contrast, if a tin coating is scratched, the exposed iron (being more reactive than tin) will start to rust rapidly at the scratch point.
8. What is meant by the electrolysis of water? Identify the gases produced at the positive and negative electrodes during this important chemical process.
Electrolysis of water is the process of using electricity to decompose water (H₂O) into its constituent elements, which are hydrogen and oxygen. A small amount of acid is usually added to the water to make it conductive.
During this process:
At the negative electrode (cathode), hydrogen gas (H₂) is produced.
At the positive electrode (anode), oxygen gas (O₂) is produced.