




An Overview of Class 11 Chemistry Quantitative Estimation Volumetric Analysis Experiment
French chemist Jean Baptiste Andre Dumas defined volumetric analysis for the first time in his experiment. He carried nitrogen from a furnace in the carbon dioxide steam and passed it into an alkali solution. The solution absorbed the carbon dioxide and nitrogen accumulated in the tube. The mass of the nitrogen was calculated from the volume present in the known pressure and temperature conditions. Hence, the volume of nitrogen is determined.
Volumetric analysis is used in biodiesel industries to find the acidity of a given sample of vegetable oil. By knowing this, the scientists calculate the amount of base that is required to neutralise the oil. Titration is also used in food industries to determine the amount of unsaturated fatty acid in the food. Example: To find Citric acid concentration in frozen orange juice, alkalinity in beverages, etc. The Karl Fischer titration method is used to analyse the amount of water present in a substance.
Table of Content
Aim
Apparatus Required
Acid-Base Titration
Procedure
Observation
Result
Aim
Determination of concentration of a given basic solution by titrating it against a standard solution of acid.
Titration of Sodium Hydroxide and Oxalic Acid.
Apparatus Required
Burette
Pipette
Conical Flask
Burette Stand
White Glaze Tile
Measuring Flask
Chemical Balance
Theory
Volumetric Analysis involves the determination of the volume of a solution of known concentration, which reacts quantitatively with the measured volume of the solution of a substance whose concentration is to be determined. The solution of known concentration is known as a standard solution. The mass of the substance dissolved in the solution's unknown concentration is calculated from the volume of the known standard solution. The reagent of known concentration is known as the titrant, and the substance of the unknown solution is known as titrand.
Acid-Base Titration: In the titration of a strong acid with a strong base, the amount of acid and base become chemically equivalent at the endpoint. Near the endpoint, the pH of the solution changed suddenly. This reaction is known as the neutralization reaction. In this process, if acid or base is added at the end, the solution becomes slightly acidic or basic. Phenolphthalein is used as an indicator in this titration method.
Titration of a weak acid (oxalic acid) with a strong base (sodium hydroxide).
(COOH)2 + 2NaOH → (COONa)2 + 2H2O
Molarity Concept:
The Titrimetric analysis is done for various types of reactions. A standard solution of acid and base is used for titration. The concentration of the solution is expressed by the molarity. The molarity of a solution is the number of moles of a solute dissolve in 1 lit of solution.
\[Molarity(M) = \dfrac{{Number\,of\,moles\,of\,solution}}{{Volume\,of\,solution\,in\,Lit}}\]
In a titration, the molarity of the solution is calculated by the formula;
[a1 M1 V1 = a2 M2 V2]
Here,
a1= Basicity of solution
M1 = Molarity of basic solution
V1 = Volume of basic solution
a2= Acidity of solution
M2= molarity of an acidic solution
V2= Volume of acidic solution
Prepare 0.1 M Standard Solution of Oxalic Acid:
Materials Required:
Measuring flask 250 ml
Funnel
Weighing tube or Watch glass
Wash bottle
Iron Stand with Ring clamp
The molar mass of oxalic acid is 126 g which means 126 g of oxalic acid is present in 1 lit of solution. It is known as one molar solution (1.0M).
To prepare 1 lit of 0.1M oxalic acid solution we require \[\dfrac{{126}}{{10}}\]g i.e. 12.6 g of hydrated oxalic acid.
To prepare 250 ml of 0.1 M oxalic acid solution, we need,
\[12.6 \times \left( {\dfrac{{250}}{{1000}}} \right) = 3.15\]gm hydrated oxalic acid
Procedure
Preparation of Oxalic Acid
First clean and dry an empty watch glass, then weigh it accurately and note the value.
Then weigh 3.150 g of oxalic acid in a watch glass and note the value.
Now transfer the oxalic acid carefully from the watch glass into a dry flask using a funnel.
Subtract the weight of the watch glass from the total weight to get the exact molarity of the oxalic acid.
Now wash the funnel many times into the flask to remove the stick particles from it with distilled water.
Make sure the water does not exceed in quality while washing the funnel.
Now swirl the measuring flask so that oxalic acid is dissolved in the water completely.
Plug the stopper into the flask and shake it well to make a uniform solution of 0.1 M oxalic acid solution.
Titration of Sodium Hydroxide and Oxalic Acid Solution
The first step of the titration is to clean the burette properly with distilled water, and then rinse it with sodium hydroxide solution. Then clamp it on the stand vertically.
Then fill the burette with sodium hydroxide solution, with the help of a funnel above the zero mark.
Remove the air by running the solution forcefully through the nozzle and then closing the nozzle.
Make sure the burette is filled up to mark, then remove the funnel.
Then note the initial reading by keeping the eye at the same level as the meniscus.
Take 10 ml of oxalic acid solution through a pipette in a dried conical flask.
Always wash the pipette before using it with the solution to be measured.
Now add 1-2 drops of phenolphthalein indicator in the conical flask, which gives it a pink colour.
Then place the flask on the glazed tile.
Now titrate the oxalic acid solution with the sodium hydroxide solution, till the pink fades.
Make sure first drop sodium hydroxide solution in a few amounts and then dropwise to get the correct reading.
Keep swirling the flask during titration.
Note down the reading properly.
Repeat the procedure until three constant readings are obtained.
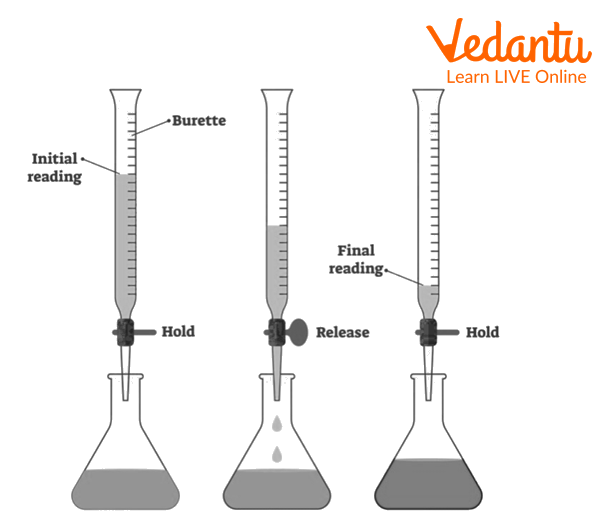
Volumetric Analysis of standard NaOH solution
Observation Table
Calculation
The molarity of NaOH is calculated by the formula;
[a1 M1 V1 = a2 M2 V2]
Where,
M1 = the molarity of an oxalic acid solution
V1 = volume of oxalic acid solution
M2= the molarity of sodium hydroxide solution
V2= the volume of NaOH solution
a1 = acidity of oxalic acid, which is 1.
a2= basicity of sodium hydroxide solution, which is 2.
M1 =0.1M
V1 = 10 mL
V2 = 10mL
The molar mass of oxalic acid = is 126 g/mol
The molar mass of NaOH is = 40 g/mol
Concentration strength in g/L = molarity Molar Mass
Result
The Concentration of sodium hydroxide is 2 g/L.
Precautions
We should rinse the burette with the solution that we are going to fill in before using it.
Always make sure that there is no air gap in the burette before titration starts.
Make sure the nozzle is perfect, there should be no leakage from it.
Note the reading on the burette, keeping your eye on the same level as the burette in the transparent solution note the reading from the lower meniscus and in the coloured solution from above the meniscus.
Do not hold the pipette at the bulb.
Make sure the pipette and burette are unbroken.
While filling the solution in the pipette, make sure the nozzle is dipped properly in the solution.
The concentration of the solution must be calculated up to the fourth decimal number for a more accurate value.
Make sure the balance is working properly and dust free.
Lab Manual Question
1. Define volumetric analysis.
Ans. Volumetric analysis is the method by which the concentration of the unknown compound is determined by titrating it with another solution of known concentration.
2. How is the strength of the solution calculated?
Ans. The strength of the solution is determined by calculating the amount of solute in grams per lit of solution. The unit of strength is g/L.
Strength=\[\dfrac{{Mass\,of\,solute\,(g)}}{{Volume\,of\,solutuion\,(L)}}\]
3. How to settle the balance before weighing the substance?
Ans. First, clean the balance with the brush or cloth properly.
Now level the balance by adjusting the levelling screws to zero.
Rotate the key arrest knob to raise the beam and see the pointer oscillates properly and equally from the zero mark.
4. What is a standard solution?
Ans. A solution of known concentration is known as the standard solution. It can be prepared by dissolving a known quantity of substance in a definite volume of solvent. It is of two types.
Primary Standard Solution: Primary standard solution is pure and cheaply available. It is highly stable. It is neither hygroscopic nor deliquescent. Examples are Mohar's salt, Oxalic acid, potassium dichromate etc.
Secondary Standard Solution: The secondary standard solutions can’t be prepared directly. Examples are potassium permanganate, sodium hydroxide, etc.
Viva Questions
1. What is the endpoint of a titration mean?
Ans. The endpoint of the titration is the point at which the reaction between the titrant and analyte completes. It is determined by using an indicator in the chemical reaction.
2. What is normality?
Ans. Normality is the number of grams equivalent to the solute dissolved in one litre of the solution. It is represented by ‘N’.
\[Normality = \dfrac{Number \ of \ grams \ equivalent \ of \ solute}{Volume \ of \ solution}\left (in \ lit \right)\]
Define molarity.
Ans. Molarity is defined as the number of grams moles of solute dissolved in one litre of the solution. It is represented by ‘M’.
\[Molarity = \dfrac{Number \ of \ gram \ moles \ of \ the \ solute} {Volume \ of \ the \ solution} \]
What is an indicator?
Ans. An indicator is a substance that undergoes a colour change at the endpoint during titration. In an acid-base titration, the endpoint is determined by an acid-base indicator. In these reactions, the indicator is either weak organic acid or weak organic base. Example- phenolphthalein.
What are the different types of acid-base titrations?
Ans. Acid-base titrations are of four types;
Strong acid-strong base titration
Strong acid-weak base titration
Weak acid-strong base titration
Weak acid-weak base titration
Define strong acid-strong base titration.
Ans. In Strong acid and strong base titration, both acid and base are strong. They neutralise each other at the endpoint. The pH value of the solution is neutral at the endpoint.
Which indicator is used in strong acid-strong base titration? Give an example.
Ans. In the strong acid-strong base titration of phenolphthalein, methyl orange indicators are used, usually to find the endpoint in the reaction.
What is molality?
Ans. Molality is defined as the number of moles of the solute dissolved in 1 kg of the solvent. It is represented by ‘m’.
\[Molality = \dfrac{Number \ of \ moles \ of \ solute}{ Mass \ of \ the \ solvent} \left ( in \ kg \right)\]
How to determine the strength of an acid or base?
Ans. The law of equivalents determines the strength of the acid or base. According to the law, the number of equivalence of the substance is titrated with an equal number of the equivalents of the titrant used.
[V1 N1 = V2 N2]
V1 and N1 are the numbers of grams of acid equivalents in V1 cm3 of N1 acid solution.
V2 and N2 are the numbers of grams equivalent to the base in V2 cm3 of the N2 basic solution.
What are the advantages of volumetric analysis?
Ans. There are the following advantages of volumetric analysis;
It is simple to perform compared to other titration methods.
It is simple and cost-friendly.
Anyone can perform it; it does not need any special skill.
Practical Questions
Apparatus required for the volumetric analysis process?
Burette,
Pipette
Conical Flask
Fractional Weight Box.
Ans. The apparatus required for volumetric analysis are Burette, pipette, conical flask, weighing Balance, fractional Weight box etc.
Analysis of the amount of substance is called?
Gravimetric Analysis
Volumetric Titration
Molarity
Normality
Ans. Analysis of the amount of substance is called volumetric analysis.
What is the use of volumetric analysis in industries?
To know the concentration of chemicals.
To determine the strength of the given solution.
To find the endpoint in a chemical reaction during titration.
All of the above.
Ans. To know the concentration of chemicals, to determine the strength of the given solution, and to find the endpoint in a chemical reaction.
Why we should not lift the weight of the analytical balance with our hands?
It gives an accurate reading.
It causes errors in the weighing.
It does not affect weighing.
None of the above.
Ans. It causes errors in the weighing.
The Secondary Standard solution definition is?
They are highly stable.
They are cheap and highly pure.
They are substances whose standard solution can not be prepared directly.
They are soluble in water.
Ans. They are substances whose standard solution can not be prepared directly.
Why is the door of the analytical balance is keep closed while measuring weight?
It can cause errors in weighing due to air
It can cause vibration in the weighing balance
It gives an accurate reading.
It does not affect balance.
Ans. The doors of the balance are kept close for an accurate reading.
What are the uses of titration?
Titration is used in laboratories to find concentrations of unknown substances.
In a titration, titrants of the known solution are added to the known quantity of analyte to check reactions.
In industries, it is used to check the concentration of the unknown substance.
All of the above.
Ans. Titration is used in laboratories, and industries to find a concentration of an unknown substance, and in titration, titrants are added to the known analyte to check reactions.
How to read the values in a colourless and transparent solution?
Read at the same level as the meniscus.
Read at the lower meniscus of the solution.
Read at the upper meniscus of the solution.
Read according to your eye view.
Ans. Read at the lower meniscus in a colourless and transparent solution.
What is not a principle of volumetric analysis?
An unknown number of chemicals must be present in the solution that needs to be examined.
A Reagent with an unknown concentration reacts with the chemical of an unknown analyte in presence of an indicator.
We should not complete titration till the endpoint.
The mole fraction determines the amount of an unknown chemical in a specific volume of solution.
Ans. We should not complete titration till the endpoint.
Why hydrochloric acid is not used in potassium permanganate titration?
It does not affect titration.
It makes the solution acidic.
It reacts with potassium permanganate and disturbs the titration.
It reduces the potassium permanganate.
Ans. It reacts with potassium permanganate and disturbs the titration.
Conclusion
From the above experiment, we can find the concentration of the sodium hydroxide solution by titrating it with oxalic acid in presence of indicator phenolphthalein using the molarity concept. We have seen that during titration, the pink colour of phenolphthalein lightens at the endpoint.
FAQs on Class 11 Chemistry Quantitative Estimation Volumetric Analysis Experiment
1. What are the essential characteristics a substance must possess to be considered an important primary standard for volumetric analysis in the CBSE Class 11 syllabus for the 2025-26 session?
For a substance to be used as a primary standard in an exam setting, it must meet several strict criteria:
It must be available in a highly pure form (99.9%+).
It should be stable at room temperature and when heated, and not be hygroscopic (absorb moisture) or efflorescent (lose water).
It should have a high molecular weight to minimise percentage errors during weighing.
It must be readily soluble in the chosen solvent, typically water.
The reaction with the titrant must be stoichiometric, rapid, and complete.
2. In the titration of potassium permanganate (KMnO₄) against oxalic acid, what is the end point and why is an external indicator not required?
In this specific titration, potassium permanganate acts as a self-indicator. The end point is visually identified by the appearance of the first permanent, light pink colour in the conical flask. This occurs because once all the oxalic acid (a reducing agent) has reacted, the very next drop of KMnO₄ (the oxidising agent) is in excess and imparts its distinct purple colour, which appears as a faint pink in the dilute solution, signalling the reaction's completion.
3. Why is rinsing the burette with the titrant solution considered a critically important step before starting a titration?
Rinsing the burette with the titrant solution is a crucial preparatory step to ensure accuracy. If the burette is wet with water from washing, the added titrant would be diluted, lowering its concentration. This would lead to a larger, incorrect titre volume being recorded to reach the end point, causing a significant error in the final molarity calculation. Rinsing removes any residual water, ensuring the concentration of the titrant inside the burette is exactly as prepared.
4. State the molarity equation, a frequently asked formula in volumetric analysis calculations.
The fundamental formula used to solve most titration problems is the molarity equation, which relates the reacting solutions based on the balanced chemical equation:
(M₁V₁) / n₁ = (M₂V₂) / n₂
Where:
M₁ and V₁ are the molarity and volume of the first substance (e.g., analyte).
M₂ and V₂ are the molarity and volume of the second substance (e.g., titrant).
n₁ and n₂ are their respective stoichiometric coefficients from the balanced equation.
5. How do common procedural flaws like parallax error and air bubbles in the burette affect the final result of a titration experiment?
These common mistakes are significant sources of error in volumetric analysis:
Parallax Error: This occurs if the burette reading is not taken at eye level. Looking from above the meniscus gives a lower reading, while looking from below gives a higher reading. This introduces a systematic error that reduces the accuracy of the titre value.
Air Bubble in Burette Tip: If an air bubble is present in the burette tip and gets dislodged during titration, the volume it occupied is incorrectly included in the titre reading. This leads to a falsely high volume reading and an inaccurate calculation of the analyte's concentration.
6. From an exam perspective, what is the important difference between the end point and the equivalence point of a titration?
While related, these two terms are not identical. The equivalence point is a theoretical point in the titration where the number of moles of the titrant added is stoichiometrically equal to the number of moles of the analyte in the solution. The end point is the experimental point where a physical change, like a colour change of an indicator, is observed. For an accurate titration, it is crucial that the end point is as close as possible to the equivalence point.
7. Why is dilute sulfuric acid (H₂SO₄) specifically added when titrating KMnO₄ against Mohr’s salt (ferrous ammonium sulfate)?
Adding dilute sulfuric acid is essential for two key reasons in this titration:
It provides the required acidic medium (H⁺ ions) for the complete reduction of permanganate ions (MnO₄⁻) to manganous ions (Mn²⁺). In a neutral or alkaline medium, KMnO₄ would be reduced to manganese dioxide (MnO₂), a brown precipitate that would obscure the end point.
It prevents the hydrolysis of ferrous sulfate in the Mohr's salt solution, which would otherwise form ferric hydroxide, thus ensuring the Fe²⁺ ions remain available to react.
8. For the important Class 11 experiment involving KMnO₄ and oxalic acid, why must the oxalic acid solution be heated to 60-70°C before titration?
The reaction between potassium permanganate and oxalic acid is exceptionally slow at room temperature. Heating the oxalic acid solution to approximately 60-70°C serves as a catalyst, significantly increasing the rate of reaction. This ensures a rapid reaction upon the addition of KMnO₄, allowing for a sharp and accurate end point. Heating above this temperature range should be avoided, as it can cause the oxalic acid to decompose into carbon dioxide, which would lead to an erroneously low titre value.
























