Science Notes for Chapter 1 Matter in Our Surroundings Class 9 - FREE PDF Download
In Chapter 1 of Class 9 Science, titled Matter in Our Surroundings, you will explore the fundamental concepts of matter and its different states. This chapter delves into the properties of solids, liquids, and gases, and how matter changes from one state to another. Our notes provide a clear and detailed explanation of these concepts, along with key points and examples to help you grasp the material effectively. Download the FREE PDF to access all the essential information and practice materials you need to understand matter in your surroundings and perform well in your studies. Visit the CBSE Class 9 Science Revision Notes and CBSE Class 9 Science Syllabus pages for more resources.
 Table of Content
Table of ContentAccess Revision Notes for Class 9 Science Chapter 1 Matter in Our Surroundings
Introduction
Everything around us is formed of the matter: a pencil, a pen, a table, the food we consume, the clothes we wear, the walls of our homes. But what is the matter?
Anything that occupies space has mass, and can be sensed by our senses is considered the matter. In other words, the term "matter" refers to all of the substances and materials that make up the cosmos.
Composition of Matter
According to ancient Indian philosophers, the matter is made up of five constituents or tattvas, according to studies found in our sacred books and scriptures.
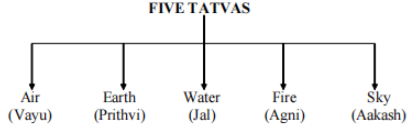
Illustration 1. How many different ways did ancient Indian philosophers classify matter?
a. $2$
b. $6$
c. $7$
d. $5$
Ans: $\left( D \right)$
Matter is made up of Particles
Now that we have defined matter let us ask ourselves the question – What is a matter made up of?
All matter comprises very small particles.
All matter can be broken up in a similar manner to get very small particles.
Hence we now conclude that all matter is made up of small particles.
Illustration 2. Which of the following are matters?
Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, the smell of perfume.
Ans: chair, air, almond, cool drink
Properties of Matter
Small particles of matter make up all matter. Some features are shared by all of these particles. A theory called Kinetic Theory of Matter explains forth these features.
Simply said, The Kinetic Theory of Matter States is a theory that describes how matter changes throughout time.
a. All matter is made up of tiny particles.
b. There is space between these particles.
c. The particles are in constant motion.
d. The particles are attracted to one another.
Particles of Matter have space between them
Small particles make up matter, and these particles have small spaces between them.
These areas are not visible to the naked eye, yet particles of other matter can pass through them without changing their volume.
Particles of Matter are continuously moving
Particles in the matter are constantly moving. Three types of motion were seen in the matter particles.
a. Translatory Motion - It occurs when particles move in straight lines and change direction without losing energy after interacting with another particle or the container's wall. When compared to liquids, translational motion is greatest in gases and least in solids.
b. Rotational motion: When particles travel about their own axis, this is known as rotational motion. This motion is comparable to the earth's rotation around its axis. In gases and liquids, the rotational motion will be quite high.
c. Vibrational Motion - When particles move back and forth around a central point. Solids have the greatest amount of motion because the particles are held in a hard framework.
Particles of Matter attract each other
1. The force with which they attract one another differs depending on the matter.
2. The force is modest in some types of materials (waste paper, matchsticks) (as we can tear or break them easily).
3. The force is large in other types of material (iron nail) (as we cannot break the nail easily).
Illustration 3. When sugar dissolves in water, what happens to it? What happens to the sugar? What does the dissolution of sugar in water tell you about the nature of matter?
Ans:
a. When sugar dissolves in water, the solid sugar crystals are broken up into microscopic particles.
b. The sugar particles interact with the water particles in the gaps between them (to form a sugar solution).
c. Sugar dissolving in water indicates that the stuff (in this case, sugar and water) is made up of minute particles. There are voids between the particles of stuff (in this case, water).
Diffusion
“The mixing and spreading out of a substance with another substance due to the movement or motion of its particles is called diffusion.”
The process of one substance diffusing into another continues until a homogenous mixture is achieved. Let's have a look at an example.
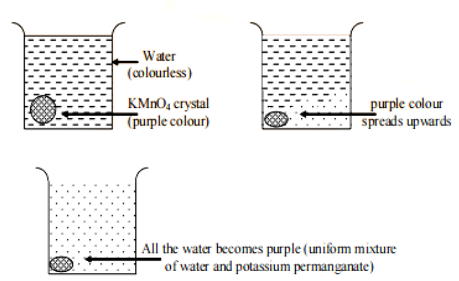
Put a crystal of potassium permanganate (purple colour) in one of the beakers that is full of water. Gradually, you'll notice that the purple-colored crystal begins to diffuse or dissolve into water, and after a while, it turns purple.
Diffusion in Gases
Gases have a very fast diffusion rate. Because gas particles move very swiftly in all directions, this is the case.
Examples \[1:\]
Even from a long distance, the smell of food being prepared in the kitchen reaches us.
The smell of hot, sizzling food reaches us even when we are a long way away, but we must approach close to get the smell of cold food.
This is because the rate of diffusion of hot gases is substantially faster than the rate of diffusion of cold gases released by cold food.
Example \[2:\]
When someone opens a bottle of perfume in one corner of a room, the scent soon travels throughout the space.
When a perfume bottle is opened, the liquid perfume soon turns into vapour (or gas).
The scent vapours flow quickly in all directions in the air, mixing with the air particles and spreading across the room.
Example
The diffusion of a strong-smelling chemical (ethyl mercaptan) found in the cooking gas into the air detects the leaking of cooking gas (LPG) in our houses.
Diffusion in Liquids
Liquid diffusion is slower than gas diffusion. This is due to the fact that particles in liquids move slower than particles in gases.
Solid in Liquid
When a crystal of potassium permanganate is placed in the bottom of a beaker of water, the purple colour of the potassium permanganate progressively spreads throughout the water.
The liquid in Liquid:
When a drop of ink is dropped into a beaker of water, the colour of the ink spreads across the entire water in the beaker; this is due to the diffusion of ink particles into water.
Gases like carbon dioxide and oxygen are necessary for aquatic plants and animals to survive. The carbon dioxide and oxygen gases in the air (or atmosphere) diffuse into and dissolve in water (ponds, lakes, and rivers). Aquatic plants use dissolved carbon dioxide to prepare food through photosynthesis, whereas aquatic animals breathe using dissolved oxygen in the water.
Diffusion in Solids
Solid-state diffusion is an extremely slow process.
Example :
If we write something on a blackboard and then leave it filthy for a long time (say, 10 to 15 days), cleaning the blackboard becomes quite tough. This is owing to the fact that certain chalk particles have dispersed into the backboard's surface.
When two metal blocks are closely linked together and left undisturbed for several years, the particles of one metal permeate into the other metal. Gases dissipate quickly. A gas's rate of diffusion is proportional to the square root of its density.
Force of Attraction (or Cohesion)
Between the particles of matter, there is an attractive force that binds them together. The force of attraction is the attraction between particles of the same substance (or cohesion).
In general, the force of attraction is greatest in solid matter particles and least in gaseous matter particles.
Illustration 4. Analyse the effects of diffusion in different states of matter, such as solid, gas, and liquid.
Ans: $\text{Solid}<\text{Liquid}<\text{Gases}$
Slow Fast Very Fast
States of Matter
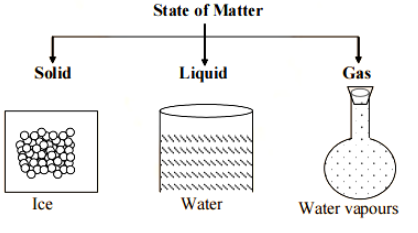
Solids have a definite volume and shape. They are more difficult to break than liquids and gases.
Liquids have a specific volume but not a specific shape. They take on the shape of the container they're housed in.
Gases do not have a defined shape or volume. They take up all of the available space and take on the shape of the container in which they are kept.
Plasma - At extremely high temperatures, the plasma state is a fused and ionic condition of matter (like the core of the sun, stars). Because it is made up of positive ions and a pool of electrons, the fused ionic mass is neutral. Around 99 per cent of the universe is made up of fused ionic matter.
S.no. | Solid | Liquid | Gas |
Solids have fixed shape and definite volume | Liquids have fixed volume but no definite shape. | Gases have no fixed volume and shape | |
Solids have high density | Liquids have a high density but less than solids. | Gases have low density. | |
Solids show only slight expansion on heating. | Liquids show slight expansion on heating but more than solids. | Gases expand considerably on heating. | |
They have slight or no compressibility. | They have slight compressibility but more than solids. | They have high compressibility. | |
Solids do not flow. | Liquids generally flow easily. | Gases flow freely. | |
They have their melting and boiling points above room temperature. | They have their melting point below room temperature. | They have their melting and boiling points both below room temperature. | |
Intermolecular forces are very strong and constituent particles are closely packed | Intermolecular forces are strong enough to keep the particles together but not strong enough to keep them in fixed positions. | Intermolecular forces are very weak and the particles are free to move. |
Illustration 5.
a. Give two reasons why wood is a solid material.
b. ‘A material has a known volume but no known shape.' Indicate if the substance is solid, liquid, or gaseous.
c. Describe the physical state of matter that can be squeezed readily.
d. ‘A substance has both a definite shape and a defined volume.' Which physical state does this statement represent? A substance has neither a fixed shape nor a fixed volume. State whether it is a solid, a liquid or a gas.
e. Give two reasons to justify that:
i. Water is a liquid at room temp.
ii. An iron almirah is solid.
Ans:
a. Wood has
i. fixed shape, and
ii. fixed volume
b. Liquid
c. Gas
d. Solid
e. Gas
i. Fixed volume but no fixed shape
ii. Fixed shape and fixed volume.
Rigid and Fluid
Rigid is a word that denotes "unbending" or "inflexible." Because it is unbending or inflexible, a stone is stiff. Fluid is defined as "a material that flows easily" and requires the use of a vessel (container) to keep it contained.
A solid is a kind of stuff that is unyielding. Solids have a tendency to keep their shape when subjected to external force due to their rigidity. As a result, rigidity is the primary distinguishing feature of solids. As a result, rigidity is the primary distinguishing feature of solids. Solids don't need to be kept in a container. Two common solids are a brick and a log of wood.
A liquid is a fluid type of stuff that fills the container's lower half. Liquids must be kept in a container because they are fluids. Because liquids have a well-defined surface, they can be stored in an open container. The liquid will not spontaneously escape from the open container. Water and milk are two prevalent liquids found in our environment.
Gas is a form of stuff that fills the entire container in which it is contained. Gases, like liquids, require a container to keep them contained. Because gas has no open surface, it must be stored in a closed container. If gas is kept in an open container, it will escape. Gases are frequently stored in airtight gas cylinders because of this. Cooking gas (LPG), for example, is stored in airtight metal cylinders. We can conclude from this discussion that fluids include both liquids and gases. Fluidity is a property of liquids and gases that allows them to flow smoothly. When exposed to external stress, liquids and gases change shape quickly due to their fluidity.
Illustration 6. Which of the following is a rigid form of matter
Alcohol
Ether
Love
Pen
Ans: Ether and alcohol
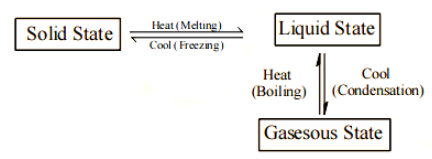
Interconversion of the state of matter
Changing the temperature, pressure, or both can cause matter to change its physical condition.
a. Melting is the transformation of a solid into a liquid.
b. Solidification is the process of turning a liquid into a solid.
c. The process of converting a liquid to a gas is known as vaporisation.
d. Condensation is the process of turning a gas into a liquid.
e. Sublimation is the process of converting a solid to a gas.
Note: While increasing pressure in gas will not change the physical condition of the gas, it will bring the particles closer together, causing the gas to liquefy.
Vaporization is promoted by lowering pressure over a liquid's surface.
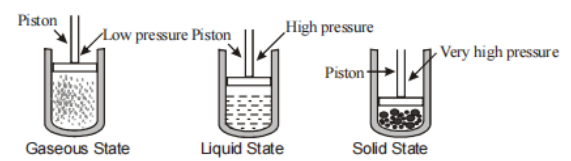
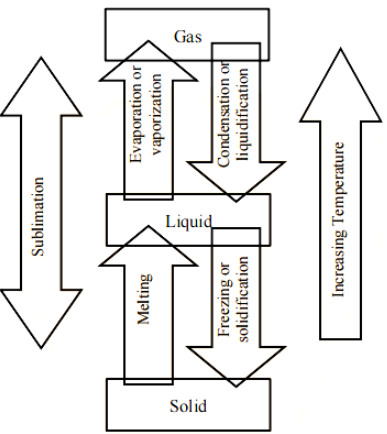
Illustration 7. When solid carbon dioxide is exposed to air, which of the following factors is responsible for the change in state?
a. Increase in pressure
b. Decrease in pressure
c. Increase in temperature
d. Decrease in temperature
Ans: $(a)$ Decrease in pressure; Increase in temperature
Effect of change of Temperature and Pressure
We can change the physical condition of matter in two ways:
a. by changing the temperature; and
b. by changing the pressure
A solid can be changed to a liquid state by raising the temperature, and a liquid may be converted to a gaseous state by lowering the temperature.
Melting (Fusion)
Melting is the transformation of a solid substance into a liquid when it is heated (or fusion).
Melting of the substance refers to the temperature at which a solid melts and transforms into a liquid at atmospheric pressure.
The heat energy in a solid substance causes its particles to vibrate more vigorously. At the melting point, a solid's particles have enough kinetic energy to overcome the strong forces of attraction that keep them in fixed places, and they break apart into small groups. And the solid transforms into a liquid.
The greater the force of attraction between the particles of a solid substance, the higher its melting point. The melting point of iron metal, for example, is extremely high (1535 degrees celsius), indicating that the force of attraction between the particles of iron is extremely strong.
Boiling (Vaporisation)
Boiling is the transformation of a liquid substance into a gas when heated rapidly.
The boiling point of a liquid is the temperature at which it boils and transforms rapidly into a gas at atmospheric pressure.
Condensation
When a gas (or vapour) is cooled sufficiently, the process of turning it into a liquid is termed condensation.
Condensation of steam occurs when steam (or water vapour) cools and converts to water (or condensation of water vapour).
It's the polar opposite of vaporisation. (Boiling)
Freezing
Freezing is the process of turning a liquid (solidification) into a solid by chilling it, the reverse of melting.
When a liquid cools, its particles lose energy, slowing its movement.
If the liquid is sufficiently chilled (to the point of freezing), each particle ceases to move and vibrates in a fixed location. The liquid freezes and solidifies at this point.
As a result of the preceding discussion, we can conclude that changing the temperature can change the state of matter.
Effect of the change in Pressure on the state of matter
Short particles separated by small distances make up matter.
Interparticle distances are exceedingly short in the solid-state.
The inter-particle distances in liquids are slightly greater than in solids.
When compared to liquids or solids, interparticle distances are greatest in the gaseous state.
As a result, it can be shown that when pressure is applied to matter, the effect on solids is insignificant because the particles are so close together.
In liquids, the effect of pressure will be minimal.
Because the interparticle distances are vast, the effect of pressure on gases will be the greatest.
As a result, when pressure is applied to gases, the particles begin to move closer together. The attractive forces between the particles increase as the particles get closer together.
This rise in attracting forces aids the gas's transition of state. When enough pressure is applied, the attraction forces build to the point where the physical state transforms from gaseous to liquid.
The reverse can be expected to happen if the pressure on a gas is decreased.
Illustration 8. Define melting process
Ans: Melting is the transformation of a solid substance into a liquid when it is heated.
Latent Heat
The heat that a substance needs to change its condition without increasing its temperature. It's called latent heat (hidden heat) because it's buried in the substance undergoing a state transition and doesn't show up as a rise in temperature.
“During a transition of state, the latent heat is used up in overcoming the force of attraction between the particles of the substance. It has no effect on the kinetic energy of the substance's particles. And since the substance's temperature does not rise.”
Illustration 9. What is the latent heat of fusion of ice?
Ans: $3.34\times {{10}^{5}}j/kg$
Latent heat of Vaporization and Fusion
There are two types of latent heat:
i. Latent heat of fusion
ii. Latent heat of vaporization
Latent heat of Vaporization
The latent heat of vaporisation is the amount of heat in Joules necessary to turn a unit quantity of 1 kg liquid into vapours without a temperature change.
Experiments have shown that it takes \[22.5\text{ }\times \text{ }{{10}^{5}}\] joules of heat to convert 1 kilogramme of water (at its boiling point, \[\text{100 }\!\!{}^\circ\!\!\text{ C}\]) to steam at the same temperature. As a result, water's latent heat of vaporisation is \[22.5\text{ }\times \text{ }{{10}^{5}}\] joules per kilogramme (or \[22.5\text{ }\times \text{ }{{10}^{5}}\]J/kg).
“If the liquid freezes to create a solid and steam condenses to form water, the substance will emit an equal amount of latent heat of fusion and vaporisation.”
The Latent Heat of Vaporization varies depending on the substance.
Latent heat of Fusion (Solid to Liquid)
It is the amount of heat in Joules required to transform one kilogramme of solid into liquid form without causing a temperature increase.
Experiments have shown that to turn 1 kilogramme of ice into water at the same temperature (\[\text{0 }\!\!{}^\circ\!\!\text{ C}\]), \[3.34\times {{10}^{5}}\] J of heat is required.
So, latent heat of fusion of ice is \[3.34\times {{10}^{5}}\] J/ Kg.
Different substances have different Latent Heat of Fusion.
Illustration 10. Why the temperature of melting ice does not rise even though heat is being supplied continuously.
Ans: Because ice is solid, its particles are attracted to one another by strong forces. These attraction forces keep the particles tightly packed in solid ice. The heat we give ice during melting is completely consumed by overcoming the forces of attraction between ice particles, causing them to loosen up and become liquid water. As a result of this heat not increasing the kinetic energy of particles, no temperature rise occurs during the melting of ice. However, once all of the ice has melted to create water, additional heating increases the kinetic energy of water particles, causing the temperature of the water to rapidly rise.
Sublimation
Sublimation is defined as the transformation of a solid into vapours on heating and back to a solid on cooling.
$\text{Solid}\rightleftarrows \text{Vapour (or Gas)}$
Ammonium chloride, iodine, camphor, naphthalene, and anthracene are some of the common substances that sublimate.
Solid carbon dioxide is yet another example of sublimation (which is commonly known as dry ice).
Carbon dioxide gas is formed when solid carbon dioxide (or dry ice) sublimates.
Illustration 11.
a. When heated, which of the following substances sublimates:
i. Sugar
ii. Urea
iii. Ice
iv. Camphor
v. Sodium Chloride
vi. Iodine
b. What happens to the heat energy that has been delivered once a solid has melted?
c. A substance's melting point is lower than room temperature. Predict the state of its physical state.
d. Is it permissible to refer to ammonia in its gaseous state as vapours?
e. What is the name of the chemical reaction that converts a solid into a gas?
f. During a substance's change of state, which of the following energy is absorbed?
i. Specific Heat
ii. Latent heat
iii. Heat of solution
g. Identify one common chemical that can change state when heated or cooled.
Ans:
a. Camphor and iodine
b. It is converted into latent heat of fusion
c. It is a liquid.
d. No, it is not
e. It is called sublimation
e. Latent heat
g. Water
Evaporation
The phenomenon of evaporation occurs when a liquid transforms to a gaseous state below its boiling point.
Water molecules are attracted to other water molecules in all directions, but the water molecules near the surface of the water are only dragged inward, which is below the water's surface.
Note: Evaporation is a phenomenon that occurs in all liquids in theory. But, in general, when we talk about evaporation, we're talking about water evaporation.
Vapour is a substance that can remain in a gaseous state at a temperature where it would ordinarily be a solid or liquid.
Examples of solids that can exist as a vapour: camphor, naphthalene
Factors Affecting Evaporation
Evaporation depends on temperature, surface area and weather conditions
a. As the quantity of water molecules at the surface grows, evaporation increases if the surface area of the water is big. As a result, more water molecules are likely to break out once they have enough kinetic energy.
b. As the temperature approaches the boiling point of water, evaporation increases. The kinetic energy of the molecules increases as the temperature rises. The extra kinetic energy required by surface molecules to break loose or evaporate is reduced as a result. As a result, evaporation increases.
c. Evaporation decreases in excessively humid weather because the air is saturated with water molecules.
d. As water evaporates, the air just above the water surface becomes saturated with water molecules.
Illustration 12. What effect does temperature and surface area have on evaporation?
Ans: Evaporation increases as the temperature and surface area increase.
Cooling Effect
How Does Evaporation Cause Cooling?
When a liquid evaporates, the energy is extracted from the liquid. As a result, it continues to cool. The liquid absorbs the energy lost by the surroundings, causing them to cool. During the summer, for example, air coolers are used to provide forced cooling.
Illustration 13. Make a note of the cooling mechanism.
Ans: As a gas particle's energy drops due to cooling, the particle's moment slows down. The particles also become significantly closer to one another, resulting in the intermolecular attraction force. The gas contracts as a result of this.
Important Topics of Class 9 Science Chapter 1 Matter in Our Surroundings you shouldn’t Miss!
These topics provide a comprehensive foundation for understanding the nature and behaviour of matter in different environments.
States of Matter: Understanding the three primary states of matter—solids, liquids, and gases—along with their properties and how they differ in terms of shape and volume.
Characteristics of Matter: Learning about the physical properties of matter, such as density, volume, and mass, and how these properties help in identifying and differentiating substances.
Change of State: Studying the processes of changing matter from one state to another, including melting, freezing, condensation, and evaporation, and the conditions under which these changes occur.
Particle Model of Matter: Exploring the concept that matter is made up of tiny particles (atoms or molecules) and how the arrangement and movement of these particles determine the state and properties of the substance.
Brownian Motion: Understanding the random movement of particles suspended in a fluid (liquid or gas) and how this motion provides evidence for the particle theory of matter.
Importance of Science Chapter 1 Matter in Our Surroundings Class 9 Notes
Class 9 science notes chapter 1 PDF provides a comprehensive understanding of Matter in Our Surroundings, which is essential for academic success and practical problem-solving.
Foundation for Further Studies: This chapter lays the groundwork for understanding more complex concepts in chemistry and physics, such as chemical reactions, thermodynamics, and states of matter in various contexts.
Understanding Matter: It helps you grasp the fundamental properties and behaviours of different states of matter—solids, liquids, and gases—along with how they change from one state to another.
Real-World Applications: Knowledge of matter and its properties is crucial for practical applications in everyday life, including understanding physical processes like cooking, refrigeration, and the behaviour of various substances in different environments.
Scientific Concepts: The chapter introduces key scientific concepts like density, volume, and the particle model of matter, which are essential for studying physical sciences and conducting experiments.
Basis for Experiments: The concepts learned in this chapter are fundamental for designing and interpreting experiments related to the properties of matter and its changes, making it a critical part of scientific education.
Tips for Learning the Class 9 Science Chapter 1 Matter in Our Surroundings
Here are some tips for learning Class 9 Science Chapter 1: Matter in Our Surroundings:
Understand Basic Concepts: Start by familiarising yourself with the different states of matter (solids, liquids, and gases) and their properties. Knowing how each state behaves will make it easier to grasp the material.
Use Visual Aids: Create diagrams or use charts to visualise the properties and changes of matter. Drawing the particle models for different states can help you better understand how particles behave.
Relate to Real Life: Connect the concepts to everyday experiences, such as observing how water changes from liquid to gas when boiled or how ice melts. This will make the concepts more relatable and easier to remember.
Perform Simple Experiments: Conduct simple experiments, like observing the evaporation of water or the condensation on a cold surface, to see the concepts in action and reinforce your learning.
Practice Key Definitions and Formulas: Memorise important definitions and formulas related to matter, such as those for density and volume. Practise solving related problems to strengthen your understanding.
Conclusion
Chapter 1 of Class 9 Science, Matter in Our Surroundings, is fundamental for understanding the basic properties and behaviours of different states of matter. By mastering concepts such as the states of matter, particle models, and changes in states, you build a solid foundation for future scientific studies. Applying real-life examples, conducting simple experiments, and reviewing key definitions and formulas will enhance your comprehension and retention. Use these notes as a valuable resource to grasp the essentials of matter and prepare effectively for exams and practical applications.
Related Study Materials for Class 9 Science Chapter 1 Matter in Our Surroundings
Students can also download additional study materials provided by Vedantu for Class 9 Science Chapter 1 Matter in Our Surroundings.
S. No | Matter in Our Surroundings Related Study Materials |
1 | Class 9 Science Matter in Our Surroundings Important Questions |
2 | |
3 | Class 9 Science Matter in Our Surroundings NCERT Exemplar Solutions |
Chapterwise Revision Notes Links for Class 9 Science
S. No | Links for Chapter-wise Class 9th Science Notes FREE PDF Download |
1 | |
2 | |
3 | |
4 | |
5 | |
6 | |
7 | |
8 | |
9 | |
10 | |
11 |
Important Study Materials for Class 9 - Science
S. No | Study Material for Class 9 - Science |
1. | |
2. | |
3. | |
4. | |
5. |
FAQs on CBSE Notes Class 9 Science Chapter 1 - Matter in Our Surroundings - 2025-26
1. What are the main topics covered in these revision notes for 'Matter in Our Surroundings'?
These notes provide a quick summary of all key topics from Chapter 1. This includes the physical nature of matter, characteristics of particles, the three states of matter (solid, liquid, gas), the effect of temperature and pressure, and concepts like evaporation, condensation, and sublimation.
2. How can these notes help me revise the Class 9 Science Chapter 1 concepts quickly?
These revision notes are designed for fast and effective learning. They present information in a concise, easy-to-understand format, using simple language and highlighting the most important definitions and formulas. This helps you recap the entire chapter in a much shorter time compared to reading the textbook from scratch.
3. What key concepts should I focus on in these notes for a last-minute revision?
For a quick revision, you should focus on:
- The differences in properties between solids, liquids, and gases.
- The processes of state change (melting, freezing, boiling, evaporation, condensation, sublimation).
- The concept of latent heat of fusion and vaporisation.
- Factors affecting the rate of evaporation.
These topics are fundamental and often appear in exams.
4. Are these notes sufficient for revising the entire chapter before my exams?
Yes, absolutely. These notes are created by subject experts to cover all the essential concepts of 'Matter in Our Surroundings' as per the CBSE syllabus. They are an excellent tool for a thorough revision to reinforce your understanding and memory before an exam.
5. How do these notes explain the connection between the particle nature of matter and the different states?
The notes clearly explain that all matter is made of tiny particles. They then connect this idea to the states of matter by focusing on two key properties: the force of attraction between particles and the kinetic energy (movement) of particles. For example, solids have strong forces and low energy, while gases have weak forces and high energy.
6. Do the revision notes provide examples to clarify complex topics like latent heat and evaporation?
Yes, the notes use simple, everyday examples to make difficult concepts easier to grasp. For instance, they explain latent heat using the example of ice melting into water at 0°C without a change in temperature. Similarly, the concept of evaporation causing cooling is explained with examples like why we feel cool after sweating.
7. Why is it important to revise the key differences between boiling and evaporation using these notes?
It's crucial because students often confuse these two processes. The notes clearly outline that boiling is a bulk phenomenon that occurs at a specific temperature, while evaporation is a surface phenomenon that can happen at any temperature. Revising this helps avoid common mistakes in exams and builds a stronger conceptual foundation.
8. How are these revision notes for 'Matter in Our Surroundings' aligned with the latest CBSE syllabus?
These notes are carefully prepared to match the CBSE Class 9 Science syllabus for the 2025-26 academic year. They cover every topic and sub-topic mentioned in the NCERT curriculum for Chapter 1, ensuring your revision is focused and relevant to what you'll be tested on.
























