
Write the statement of Kelvin-Planck and Clausius of the second law of thermodynamics and show the equivalence of the two statements.
Answer
521.1k+ views
Hint: The second law of thermodynamics states that the entropy of an isolated system is always increasing or is constant only if the process is reversible or in thermal equilibrium. State the statements of Kelvin-Planck and Clausius and show that if one of the statements is violated, another one is also violated.
Complete step by step answer:
According to the Kelvin-planck statement, “it is impossible to make a heat engine, which absorbs energy in the form of heat from a single heat reservoir and delivers an equivalent amount of work. This implies that it is impossible to build a heat engine with hundred-percent thermal efficiency.”
According to Clausius, “It is not possible to design a device which operates on a cycle and results only in transfer of heat, without doing any other work, from a cooler body to a hotter body.”
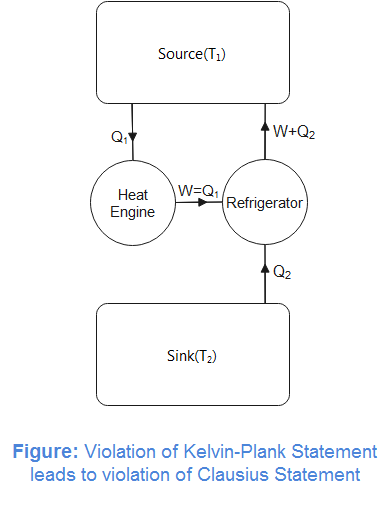
Let us assume an engine violating the Kelvin-Planck statement. That is, it extracts heat from the hot reservoir and converts it completely into work and no heat is rejected at lower temperature. We also assume that the work done by this hypothetical heat engine is used to drive a refrigerator between the same source and sink. We assume that the refrigerator completes in the same period that of the heat engine. We can now note that the heat engine and refrigerator paired between the source and sink transfer heat from lower temperature (sink) to higher temperature (source) which violates Clausius statement of the second law of thermodynamics. Now, it can be concluded that the two statements of the second law of thermodynamics are equivalent.
Note: The second law of thermodynamics states that the entropy of an isolated system is always increasing or is constant only if the process is reversible or in thermal equilibrium. Since all the natural processes are irreversible, the entropy of the universe is always increasing.
Complete step by step answer:
According to the Kelvin-planck statement, “it is impossible to make a heat engine, which absorbs energy in the form of heat from a single heat reservoir and delivers an equivalent amount of work. This implies that it is impossible to build a heat engine with hundred-percent thermal efficiency.”
According to Clausius, “It is not possible to design a device which operates on a cycle and results only in transfer of heat, without doing any other work, from a cooler body to a hotter body.”

Let us assume an engine violating the Kelvin-Planck statement. That is, it extracts heat from the hot reservoir and converts it completely into work and no heat is rejected at lower temperature. We also assume that the work done by this hypothetical heat engine is used to drive a refrigerator between the same source and sink. We assume that the refrigerator completes in the same period that of the heat engine. We can now note that the heat engine and refrigerator paired between the source and sink transfer heat from lower temperature (sink) to higher temperature (source) which violates Clausius statement of the second law of thermodynamics. Now, it can be concluded that the two statements of the second law of thermodynamics are equivalent.
Note: The second law of thermodynamics states that the entropy of an isolated system is always increasing or is constant only if the process is reversible or in thermal equilibrium. Since all the natural processes are irreversible, the entropy of the universe is always increasing.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




