
Write the postulates of Dalton’s Atomic theory.
Answer
585.3k+ views
Hint: Dalton’s atomic theory was the first complete attempt to describe all matter in terms of atoms and their properties. It provides conservation law.
Complete step by step solution:
Postulates:
1. The matter is made up of invisible particles known as atoms.
2. Atoms of the same element are similar in shape and mass, but differ from the atom of other elements.
3. Atoms cannot be created or destroyed.
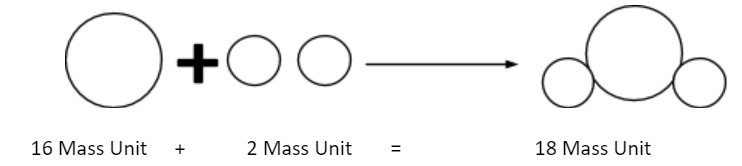
4. Atoms of different elements may combine with each other in a fixed, simple, whole number ratio to form compound atoms.
5. Atoms of the same element can combine in more than one ratio to form two or more compounds.
6. The atom is the smallest unit of matter that can take part in a chemical reaction.
Note: However this theory is not entirely free of limitations, as an atom can be subdivided into electrons, protons and neutrons. Atoms of two different elements differ in mass, size and many other chemical or physical properties. However, this is not correct for all situations. Some postulates of this theory remain valid even in today’s modern chemical thoughts. It forms the base for modern atomic theories.
Complete step by step solution:
Postulates:
1. The matter is made up of invisible particles known as atoms.
2. Atoms of the same element are similar in shape and mass, but differ from the atom of other elements.
3. Atoms cannot be created or destroyed.
4. Atoms of different elements may combine with each other in a fixed, simple, whole number ratio to form compound atoms.
5. Atoms of the same element can combine in more than one ratio to form two or more compounds.
6. The atom is the smallest unit of matter that can take part in a chemical reaction.

Note: However this theory is not entirely free of limitations, as an atom can be subdivided into electrons, protons and neutrons. Atoms of two different elements differ in mass, size and many other chemical or physical properties. However, this is not correct for all situations. Some postulates of this theory remain valid even in today’s modern chemical thoughts. It forms the base for modern atomic theories.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




