
The mass of electron, proton and neutron in grams are:
- • $1.108 \times {10^{ - 28}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{ - 24}}$
• $9.108 \times {10^{28}}$, $1.6726 \times {10^{24}}$, $1.675 \times {10^{24}}$
• $9.108 \times {10^{ - 24}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{24}}$
• $9.108 \times {10^{ - 28}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{ - 24}}$
- • $1.108 \times {10^{ - 28}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{ - 24}}$
Answer
612k+ views
Hint: - We must understand the position of electrons, neutrons and protons. For this we must know the structure of an atom,for answers can start here.
Complete answer:
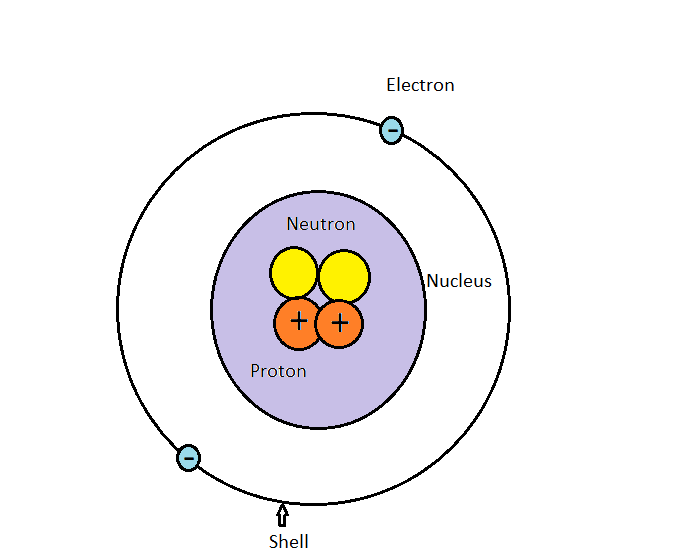
An atom consists of a nucleus in which there is proton and neutron. Protons are positively charged and neutrons are having no charge. Around nucleus electrons rotate in an orbit and it is known as a shell. Electrons are negatively charged. Atoms have different properties based on the arrangement and number of their basic particles.
An electron is a tiny particle with a mass of $9.108 \times {10^{ - 28}}$ g and a negative charge. All neutral atoms contain electrons. The mass of a proton is $1.6726 \times {10^{ - 24}}$ g, or about 1836 times the mass of an electron. The proton carries a positive electrical charge, that is equal in magnitude to the charge of the electron but opposite in sign. Mass of a neutron is $1.675 \times {10^{ - 24}}$ g. It is very close to that of the proton. A neutron carries no charge.
So, our option D is the correct answer. As per this the mass of electron, proton and neutron in grams are:$9.108 \times {10^{ - 28}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{ - 24}}$
Note: - In these types of questions we must understand the structure of the atom and the position of a particular element in the periodic table. Structure of an atom is similar to the structure of the solar system in which the sun is stationary and earth revolves around the sun in a fixed orbit.
Complete answer:

An atom consists of a nucleus in which there is proton and neutron. Protons are positively charged and neutrons are having no charge. Around nucleus electrons rotate in an orbit and it is known as a shell. Electrons are negatively charged. Atoms have different properties based on the arrangement and number of their basic particles.
An electron is a tiny particle with a mass of $9.108 \times {10^{ - 28}}$ g and a negative charge. All neutral atoms contain electrons. The mass of a proton is $1.6726 \times {10^{ - 24}}$ g, or about 1836 times the mass of an electron. The proton carries a positive electrical charge, that is equal in magnitude to the charge of the electron but opposite in sign. Mass of a neutron is $1.675 \times {10^{ - 24}}$ g. It is very close to that of the proton. A neutron carries no charge.
So, our option D is the correct answer. As per this the mass of electron, proton and neutron in grams are:$9.108 \times {10^{ - 28}}$, $1.6726 \times {10^{ - 24}}$, $1.675 \times {10^{ - 24}}$
Note: - In these types of questions we must understand the structure of the atom and the position of a particular element in the periodic table. Structure of an atom is similar to the structure of the solar system in which the sun is stationary and earth revolves around the sun in a fixed orbit.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




