
The correct structure of tribromo octaoxide is :
a.)
b.)
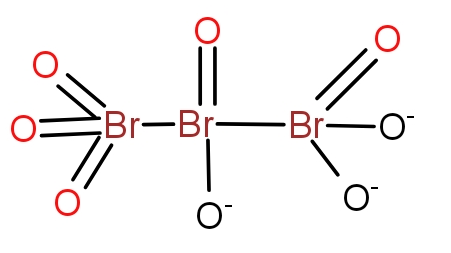
c.)
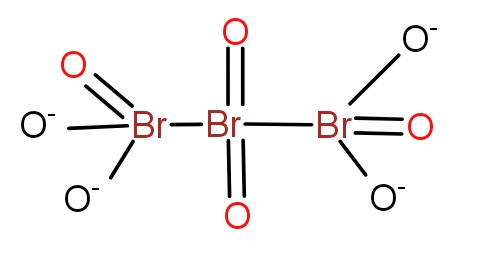
d.)
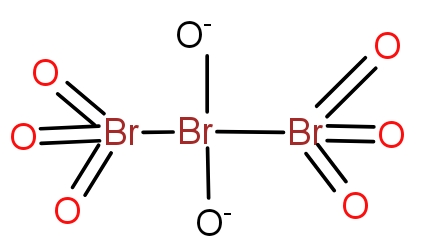




Answer
577.8k+ views
Hint:. The tribromo octaoxide is a neutral molecule and does not contain any charge. The structure of molecules contain all oxygen atoms bonded with double bonds with bromine atoms. The molecule contains three bromine atoms and eight oxygen atoms.
Complete step by step answer:
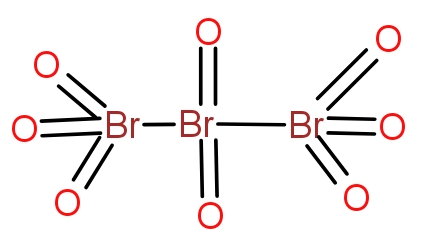
Bromine combines with oxygen to give a variety of oxides. The tribromo octaoxide is the oxide of bromine that contain three bromine atoms and eight oxygen atoms. The structure of the oxide is as-
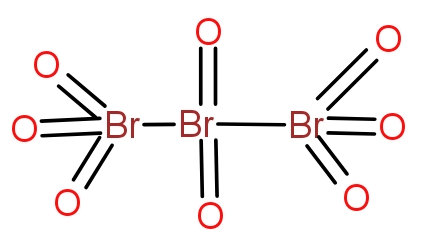
The two terminal bromine atoms bond with three oxygen atoms and a Br-Br bond. The central bromine atom forms a double bond with two oxygen atoms and a single bond with two bromine atoms. The molecule is neutral overall and does not contain any charge.
So, the correct answer is “Option A”.
Additional Information:
Bromine is a red brown liquid that evaporates very easily as the cap of the bottle is opened. It is corrosive and its vapours can irritate eyes and throat. The bromine higher oxides are least stable if we compare them with other halide oxides because these lack high polarity and multiple bond formation that involve the use of d orbitals. The chlorine oxides form multiple bonds with the help of d- orbitals.
Note: Bromine can form a variety of oxides. Some are dibromine monoxide, bromine dioxide, dibromine trioxide and dibromine pentoxide etc. Even a number of ions are bromine oxides like hypobromite, bromite, bromate and perbromate etc.
Complete step by step answer:
Bromine combines with oxygen to give a variety of oxides. The tribromo octaoxide is the oxide of bromine that contain three bromine atoms and eight oxygen atoms. The structure of the oxide is as-

The two terminal bromine atoms bond with three oxygen atoms and a Br-Br bond. The central bromine atom forms a double bond with two oxygen atoms and a single bond with two bromine atoms. The molecule is neutral overall and does not contain any charge.
So, the correct answer is “Option A”.
Additional Information:
Bromine is a red brown liquid that evaporates very easily as the cap of the bottle is opened. It is corrosive and its vapours can irritate eyes and throat. The bromine higher oxides are least stable if we compare them with other halide oxides because these lack high polarity and multiple bond formation that involve the use of d orbitals. The chlorine oxides form multiple bonds with the help of d- orbitals.
Note: Bromine can form a variety of oxides. Some are dibromine monoxide, bromine dioxide, dibromine trioxide and dibromine pentoxide etc. Even a number of ions are bromine oxides like hypobromite, bromite, bromate and perbromate etc.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




