
Statement: Rhombohedral, triclinic and hexagonal are the unit cells which have only primitive arrangement possible.
State whether the given statement is true or false.
A.True
B.False
Answer
581.4k+ views
Hint: A unit cell is the smallest component of a crystal lattice that displays the whole crystal's three-dimensional pattern. It is possible to conceive of a crystal as the same unit cell replicated in three dimensions over and over. There are various types of arrangement in a unit cell, primitive arrangement is one among that.
Complete step by step answer:
Let’s first learn about these rhombohedral, triclinic, and hexagonal unit cells.
A rotation axis of order three along the unit cell's body diagonal, limits all the sides to be equal in length and all the angles to be equal. The primitive rhombohedral is a unit cell with these axes.
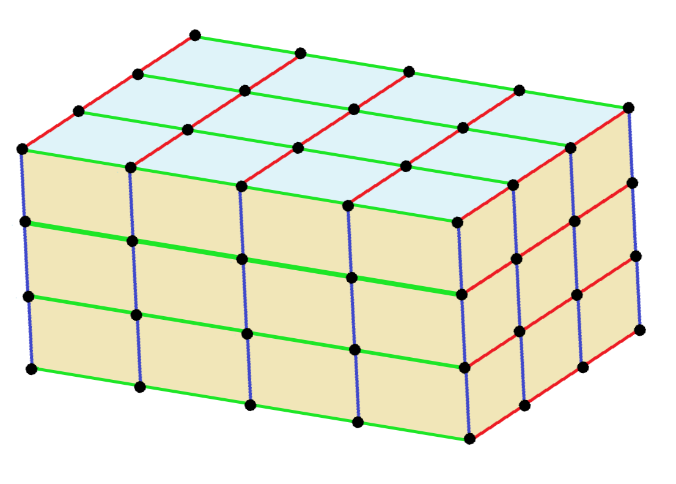
Now, a triclinic unit cell can be described as follows; The crystal is described in the triclinic system by vectors of unequal length. Furthermore, all the angles of these vectors must be distinct and may not be the right angle.
Then, the hexagonal unit cell is the one in which two sides are of equal length. Here, an atom is also centered inside the unit cell, and the unit cell is extended into two atoms whose center lies outside the unit cell. The composition of the solid, which can be considered as an almost infinite repeat of the unit cell, is fully represented by this unit cell.
WSO, what are primitive unit cells? In mathematics, biology, mineralogy-especially crystallography, and solid-state physics, a primitive cell also known as a primitive unit cell is a minimum-volume unit cell, referring to a single lattice point of a structure with discrete translation symmetry.
Now, coming to the question;
There are seven basic crystal structures; cubic, tetragonal, orthorhombic, hexagonal, monoclinic, rhombohedral, and triclinic. They vary in the way they organize their crystallographic axes and angles.
Now, let's look into the arrangements possible;
Primitive, body-centered, and face-centric configurations are possible for cubic unit cells.
Primitive and body-centered configurations are possible for tetragonal unit cells.
Primitive, body-centered, face-centered, and end-centered configurations are possible for orthorhombic unit cells.
In the case of monoclinic unit cells, end centered and primitive arrangement are possible.
So, from the above explanations, it is clear that rhombohedral, triclinic, and hexagonal are the unit cells which have only primitive arrangement possible.
So, the above statement is true.
Note:
The atoms are found in the primitive cubic unit cell only at the corners. Each atom in the corner is distributed between eight adjacent unit cells. In the same layer, there are four unit cells and four in the upper or lower layer.
Complete step by step answer:
Let’s first learn about these rhombohedral, triclinic, and hexagonal unit cells.
A rotation axis of order three along the unit cell's body diagonal, limits all the sides to be equal in length and all the angles to be equal. The primitive rhombohedral is a unit cell with these axes.

Now, a triclinic unit cell can be described as follows; The crystal is described in the triclinic system by vectors of unequal length. Furthermore, all the angles of these vectors must be distinct and may not be the right angle.

Then, the hexagonal unit cell is the one in which two sides are of equal length. Here, an atom is also centered inside the unit cell, and the unit cell is extended into two atoms whose center lies outside the unit cell. The composition of the solid, which can be considered as an almost infinite repeat of the unit cell, is fully represented by this unit cell.

WSO, what are primitive unit cells? In mathematics, biology, mineralogy-especially crystallography, and solid-state physics, a primitive cell also known as a primitive unit cell is a minimum-volume unit cell, referring to a single lattice point of a structure with discrete translation symmetry.
Now, coming to the question;
There are seven basic crystal structures; cubic, tetragonal, orthorhombic, hexagonal, monoclinic, rhombohedral, and triclinic. They vary in the way they organize their crystallographic axes and angles.
Now, let's look into the arrangements possible;
Primitive, body-centered, and face-centric configurations are possible for cubic unit cells.
Primitive and body-centered configurations are possible for tetragonal unit cells.
Primitive, body-centered, face-centered, and end-centered configurations are possible for orthorhombic unit cells.
In the case of monoclinic unit cells, end centered and primitive arrangement are possible.
So, from the above explanations, it is clear that rhombohedral, triclinic, and hexagonal are the unit cells which have only primitive arrangement possible.
So, the above statement is true.
Note:
The atoms are found in the primitive cubic unit cell only at the corners. Each atom in the corner is distributed between eight adjacent unit cells. In the same layer, there are four unit cells and four in the upper or lower layer.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




