
The rise in the water level in a capillary tube of radius 0.07 cm when dipped vertically in a beaker containing water of surface tension 0.07 \[N{m^{ - 1}}\] is $(g = 10m{s^{ - 2}})$
(A) 2 cm
(B) 4 cm
(C) 1.5 cm
(D) 3 cm
Answer
233.1k+ views
Hint When a capillary tube is inserted in water or any other liquid. It experiences capillary rise or fall depending on the density of the liquid. We know that the rise in height of the capillary is directly proportional to surface tension and angle of contact. Also, it is inversely proportional to the radius of the tube, the density of the liquid, and the acceleration due to gravity.
Complete step-by-step answer:
We are given that the radius of the capillary tube is, $r = 0.07cm = 0.07 \times {10^{ - 2}}m$
The surface tension of water is also given, $S = 0.07N{m^{ - 1}}$ .
We know that the density of water is, $\rho = {10^3}kg{m^{ - 3}}$ .
The angle of contact for water is zero degrees.
Using the expression for the rise in the capillary tube : $h = \dfrac{{2S\cos \theta }}{{r\rho g}}$
Where h is the rise of a liquid in a capillary tube
S is the surface tension of the liquid used in the capillary tube
$\theta $ is the angle of contact
r is the radius of the capillary tube
$\rho $ is the density of the liquid
and g is the acceleration due to gravity
Now, substituting all the given values in the expression for h, we get
$
\Rightarrow h = \dfrac{{2 \times 0.07 \times \cos 0^\circ }}{{0.07 \times {{10}^{ - 2}} \times {{10}^3} \times 10}} \\
\Rightarrow h = 2 \times {10^{ - 2}}m \\
\Rightarrow h = 2cm \\
$
Therefore, option (A) is correct.
Note The angle of contact is the angle that a perpendicular to the walls of the capillary makes with the meniscus of the liquid at the point of contact.
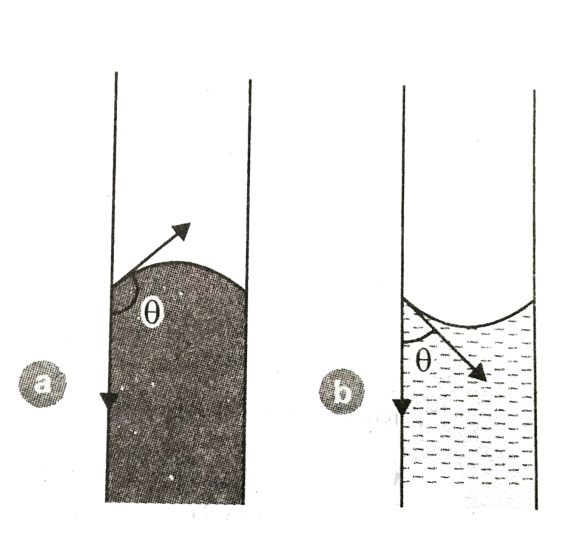
This is a diagram of two liquids where liquid(a) has a convex meniscus $(\theta > 90^\circ )$ and liquid(b) has a concave meniscus $(\theta > 90^\circ )$. For water, the meniscus is flat. Therefore, the angle of contact is zero for water.
Complete step-by-step answer:
We are given that the radius of the capillary tube is, $r = 0.07cm = 0.07 \times {10^{ - 2}}m$
The surface tension of water is also given, $S = 0.07N{m^{ - 1}}$ .
We know that the density of water is, $\rho = {10^3}kg{m^{ - 3}}$ .
The angle of contact for water is zero degrees.
Using the expression for the rise in the capillary tube : $h = \dfrac{{2S\cos \theta }}{{r\rho g}}$
Where h is the rise of a liquid in a capillary tube
S is the surface tension of the liquid used in the capillary tube
$\theta $ is the angle of contact
r is the radius of the capillary tube
$\rho $ is the density of the liquid
and g is the acceleration due to gravity
Now, substituting all the given values in the expression for h, we get
$
\Rightarrow h = \dfrac{{2 \times 0.07 \times \cos 0^\circ }}{{0.07 \times {{10}^{ - 2}} \times {{10}^3} \times 10}} \\
\Rightarrow h = 2 \times {10^{ - 2}}m \\
\Rightarrow h = 2cm \\
$
Therefore, option (A) is correct.
Note The angle of contact is the angle that a perpendicular to the walls of the capillary makes with the meniscus of the liquid at the point of contact.

This is a diagram of two liquids where liquid(a) has a convex meniscus $(\theta > 90^\circ )$ and liquid(b) has a concave meniscus $(\theta > 90^\circ )$. For water, the meniscus is flat. Therefore, the angle of contact is zero for water.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding Uniform Acceleration in Physics

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Laws of Motion Class 11 Physics Chapter 4 CBSE Notes - 2025-26

Waves Class 11 Physics Chapter 14 CBSE Notes - 2025-26

Mechanical Properties of Fluids Class 11 Physics Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Physics Chapter 11 CBSE Notes - 2025-26

Units And Measurements Class 11 Physics Chapter 1 CBSE Notes - 2025-26




