




How Does a Manometer Differ from a Barometer?
Barometer and manometer are fundamental devices used for pressure measurement in physics. A barometer determines atmospheric pressure, while a manometer measures the pressure of fluids or gases with reference to atmospheric pressure. Understanding their construction and principles is essential for mastering fluid mechanics in JEE Main Physics.
Definition and Function of Barometer and Manometer
A barometer is a precision instrument designed to measure atmospheric pressure. Atmospheric pressure corresponds to the force exerted by air on a unit area. A manometer measures the pressure of a given gas or liquid in a vessel, often relative to atmospheric pressure. The applications and construction of these instruments are distinct.
Construction and Types of Barometer
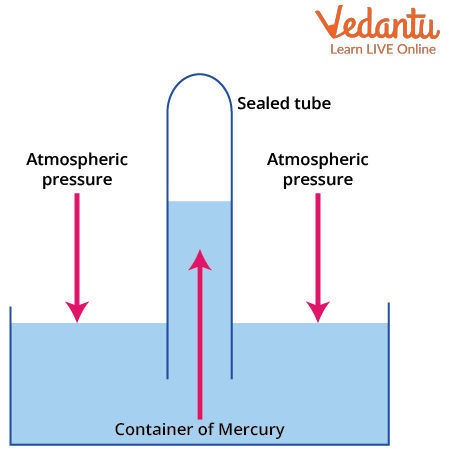
The most common type is the mercury barometer, which consists of an inverted glass tube filled with mercury and placed in a mercury reservoir. The vertical height of mercury in the tube is a direct measure of atmospheric pressure. Barometers help in various fields, including meteorology.
An aneroid barometer contains a metallic vacuum capsule made from copper-beryllium alloy. This capsule expands or contracts with changes in atmospheric pressure. Mechanical levers transfer this capsule movement to a pointer on a calibrated scale, providing a pressure reading.
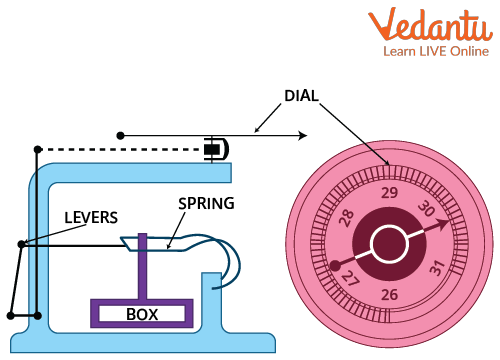
Working Principle of Barometer
The principle underlying a mercury barometer is the balance between atmospheric pressure and the pressure of a column of mercury. In equilibrium, the pressure due to the mercury column equals atmospheric pressure acting on the reservoir surface. The top of the mercury column inside the tube contains a vacuum, so the pressure is zero there.
Mathematically, atmospheric pressure is given by:
$P_{\text{atm}} = h \cdot \rho \cdot g$
Here, $h$ is the height of the mercury column, $\rho$ is the density of mercury, and $g$ is the acceleration due to gravity.
Construction and Types of Manometer
A manometer is typically constructed as a U-shaped tube containing a suitable liquid such as mercury, water, or oil. The pressure to be measured is applied to one limb, while the other limb may be open to the atmosphere or connected to another pressure source. Manometers can be classified as U-tube, well-type, or inclined types depending on application requirements.
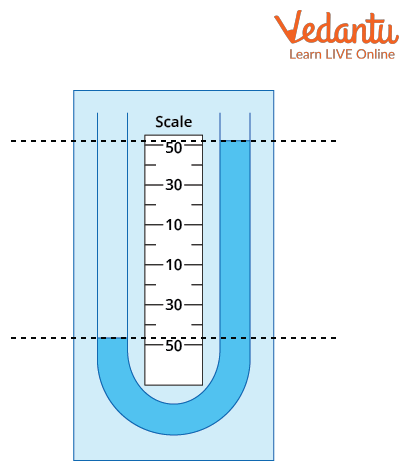
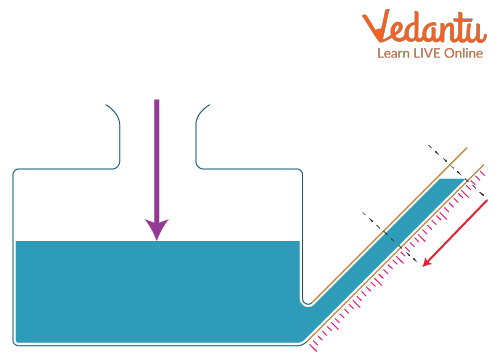
Working Principle of Manometer
A manometer operates on the hydrostatic principle. A pressure difference between its two limbs leads to a height difference ($h$) in the liquid columns. The pressure difference can be calculated using the relation:
$\Delta P = h \cdot \rho \cdot g$
For a U-tube manometer, if one side is open to the atmosphere and the other side is connected to a gas container, the pressure difference between the gas and the atmosphere determines the difference in mercury levels.
Inclined manometers increase measurement accuracy for small pressure differences by extending the length over which the liquid moves for a given pressure.
Differences Between Barometer and Manometer
Barometers and manometers differ in construction, function, and application. The following table summarizes the key distinctions.
| Barometer | Manometer |
|---|---|
| Measures atmospheric pressure | Measures fluid/gas pressure |
| Types: Mercury, aneroid | Types: U-tube, well, inclined |
| Not suited for pipe/gas pressure | Suitable for container/pipe pressure |
| Used in weather prediction | Used in laboratories and industries |
Selection of Fluids in Barometers and Manometers
Mercury is the preferred fluid for both barometers and manometers due to its high density and low vapor pressure. A high-density liquid produces a shorter column for the same pressure, making instruments compact. Similarly, non-evaporative fluids are chosen to avoid measurement inaccuracies.
Applications of Barometer and Manometer
Barometers are primarily used for determining atmospheric pressure, essential in meteorology, altitude measurement, and aircraft instrumentation. Manometers are utilized for measuring pressure differences in laboratories, gas supply systems, and industrial applications involving fluid flow.
Relevant Formulas and Example
Key formulas for pressure measurement using these instruments are directly derived from hydrostatics, as discussed in Liquid State of Matter. Calculations require values of liquid density, gravity, and measured height difference.
- Total pressure: $P = h \cdot \rho \cdot g$
- Difference in pressure for manometer: $\Delta P = h \cdot \rho \cdot g$
For example, if a mercury barometer shows a column height of $76$ cm, the atmospheric pressure is $P_{\text{atm}} = 0.76 \times 13,600 \times 9.8$ Pa.
Factors Affecting Measurements in Barometers and Manometers
Accuracy in measurement depends on correct fluid selection, temperature stability, and proper calibration. Mercury is ideal due to its high density and non-wettability. Water is seldom used in barometers, as it would require impractically tall columns.
Pressure variations with altitude and atmospheric changes can be quantitatively studied using these devices, as outlined in Hydrostatics.
Relationship with Other Physics Concepts
Understanding the operation of barometer and manometer is closely related to concepts such as fluid pressure, thrust, and thermal equilibrium. Studying Fluid Pressure and Difference Between Pressure and Thrust will reinforce core principles.
In addition, knowledge of the Properties of Solids and Liquids and Thermal Equilibrium supports a deeper understanding of instrument behavior under different conditions.
FAQs on Understanding Barometers and Manometers: Principles, Differences, and Uses
1. What is a barometer and what is it used for?
A barometer is an instrument used to measure atmospheric pressure. Understanding atmospheric pressure is important for weather prediction and several scientific experiments.
- Barometers help forecast weather by indicating changes in air pressure.
- They are used in meteorology and physics lessons for practical observation of pressure variations.
- Common types include mercury barometer and aneroid barometer.
2. What is a manometer and how does it work?
A manometer is a device used to measure the pressure of gases or liquids. It compares the pressure of a fluid with atmospheric pressure or another reference point.
- Simple U-tube manometers use a liquid column (often mercury or water) to show pressure difference.
- One end is open to the atmosphere and the other is connected to the system whose pressure needs to be measured.
- The difference in liquid levels relates directly to the pressure difference.
3. What is the difference between a barometer and a manometer?
The main difference is that a barometer measures atmospheric pressure, while a manometer measures the pressure of a contained fluid or gas.
- Barometer: Measures only atmospheric pressure.
- Manometer: Used for measuring the pressure of gases or liquids in a vessel.
- Both instruments use the concept of liquid columns and pressure differences in their functioning.
4. How does a mercury barometer measure atmospheric pressure?
A mercury barometer measures atmospheric pressure by the height of mercury in an inverted tube.
- One end of the tube is sealed, the other open and resting in a mercury reservoir.
- Atmospheric pressure pushes the mercury up the tube; height of the mercury column indicates the pressure.
- The normal atmospheric pressure supports a mercury column about 76 cm (760 mm) high at sea level.
5. What are the different types of manometers?
Manometers come in several types depending on application and design.
- U-tube manometer (simple, uses liquid columns)
- Differential manometer (measures pressure difference between two points)
- Inclined manometer (more sensitive for small pressures)
- Digital manometers (electronically display pressure values)
6. Why is mercury commonly used in barometers and manometers?
Mercury is used because of its high density, non-evaporative nature, and low vapor pressure.
- Its density allows for compact instruments with short columns.
- It does not evaporate easily under normal conditions.
- The bright visibility and non-wettability with glass make it ideal for accurate measurements.
7. What are the applications of a manometer?
Manometers are widely used to measure pressure in laboratories, industries, and for scientific purposes.
- Monitoring gas pressures in pipelines.
- Calibrating and testing pressure gauges.
- Used in experiments involving Boyle's Law and atmospheric studies.
- Essential in air flow and ventilation system analysis.
8. How do you read the pressure from a U-tube manometer?
The pressure difference is calculated from the height difference of the liquid columns.
- Measure the difference in height between the two arms of the U-tube.
- If the arms are at the same height, the pressure is equal to atmospheric pressure.
- If one arm rises, the pressure at the other side is higher by 'hρg', where:
- h: Height difference
- ρ (rho): Density of manometric liquid
- g: Acceleration due to gravity
9. What are the advantages and disadvantages of using an aneroid barometer over a mercury barometer?
An aneroid barometer is portable and safer but may be less accurate than a mercury barometer.
- Advantages:
- Portable, compact and durable
- No risk of mercury poisoning
- Easy to handle and transport
- Disadvantages:
- Can become inaccurate over time (needs calibration)
- Less precise for scientific experiments compared to mercury barometers
10. Can a barometer be used as an altimeter? How?
Yes, a barometer can be used as an altimeter because atmospheric pressure decreases with height.
- By measuring the drop in air pressure, you can estimate altitude above sea level.
- This principle is used in altimeter instruments for aviation and hiking.
- Calibrating the barometer for altitude readings allows for approximate elevation measurements.
11. What precautions should be taken while using a mercury barometer in the laboratory?
Safety guidelines should be strictly followed while handling a mercury barometer.
- Handle with care to prevent mercury spills.
- Use gloves and protective gear.
- Mop up mercury spills with proper materials (never with bare hands).
- Well-ventilated area is preferred due to mercury vapor risk.
- Follow disposal protocols as per lab safety rules.
























