




Concepts of Redox Reactions and Electrochemistry for JEE Main Chemistry
Both topics, Redox Reactions and Electrochemistry, are very important topics of chemistry. Redox reactions are oxidation and reduction reactions that define all chemical reactions that lower or raise the oxidation number. Electrochemistry, on the other hand, is the study of producing electricity from energy produced during spontaneous chemical reactions. It also deals with the utilisation of energy in non-spontaneous chemical transformations which are some of the primary applications of redox reactions.
This article includes the topics related to the redox reaction such as oxidation and reduction reaction, types of oxidation reaction i.e., combination, decomposition, displacement, and disproportionation reactions. In addition, the electrode and redox couple processes are noted here. Redox reactions also have a wide range of applications in the study of electrode and cell processes. This article also describes several redox reaction laws. It also discusses how to balance a redox reaction and the importance of titration in redox reactions.
The electrochemistry revision notes are also covered in detail in this article. The chapter is particularly significant in terms of both practical and theoretical implications. Most notably, electrochemical processes are used to produce a large number of metals and compounds. For example, batteries and fuel cells are responsible for converting chemical energy into electrical energy.
The reactions that emerge from electrochemical processes are both energy efficient and environment friendly. As a result, the importance of electrochemistry increases as it aids in the development of new revolutionary technologies that are environmentally benign. Also, electrochemistry allows sensory impulses to be transmitted from the brain to the cells and vice versa.
Without a doubt, both redox reactions and electrochemistry are large and interdisciplinary topics, and both are important for JEE and NEET exams.
JEE Main Chemistry Chapters 2026
Important Topics of Redox Reactions and Electrochemistry Chapter
Electrical Conductors
Electrolytic Conductance, Molar Conductance And Specific Conductance
Kohlrausch Law
Faraday's Laws of Electrolysis
Electrolysis and Electroplating
Cell Potential and Nernst Equation
Electronic Concepts of Oxidation and Reduction, Redox Reactions, Oxidation Number, and Balancing of Redox Reactions
Redox (reduction-oxidation) reactions are fundamental in chemistry, involving the exchange of electrons between reactants. To understand them, one must grasp key concepts such as oxidation and reduction, oxidation numbers, and how to balance redox reactions.
Oxidation and Reduction: In a redox reaction, oxidation is the loss of electrons, while reduction is the gain of electrons. Oxidizing agents accept electrons (are reduced), while reducing agents donate electrons (are oxidized).
Redox Reactions: Redox reactions are characterized by the transfer of electrons. For example, in the reaction between hydrogen and oxygen to form water, hydrogen is oxidized (loses electrons), while oxygen is reduced (gains electrons).
Oxidation Number: Oxidation numbers (or oxidation states) are assigned to atoms in a compound to represent the apparent charge of an atom in a molecule. Rules for assigning oxidation numbers include:
The oxidation number of an element in its elemental form is zero.
In compounds, hydrogen is typically +1, oxygen is -2, and the sum of oxidation numbers in a neutral compound is zero.
In polyatomic ions, the sum of oxidation numbers is equal to the charge of the ion.
Balancing Redox Reactions: Balancing redox reactions involves ensuring that the number of electrons lost in oxidation equals the number gained in reduction. Common methods include the half-reaction method and the oxidation number method.
In the half-reaction method, the reaction is divided into two half-reactions, one for oxidation and one for reduction. Balance the atoms and charges in each half-reaction, then combine them to form the balanced overall equation.
In the oxidation number method, changes in oxidation numbers for each element are tracked. Adjust coefficients to ensure that the electrons lost in oxidation are gained in reduction.
Balancing redox reactions is a critical skill in analytical chemistry, electrochemistry, and many industrial processes. Understanding the electronic concepts of redox reactions and mastering the rules for assigning oxidation numbers are essential for successfully balancing these reactions, making it a crucial aspect of chemical knowledge and problem-solving.
Redox Reactions and Electrochemistry Important Concept for JEE Main
Electrical Conductor
Conductors or electrical conductors are materials that allow an electric current to travel through them.
Non-conductors or insulators, on the other hand, are substances that do not enable electricity to pass through them. Rubber, wood, paper, and all non-metals except carbon are the examples of non-conductors.
There are two types of conductors: (i) metallic conductors and (ii) electrolytic conductors.
Electrolytic Conductance
Free ions are present in molten electrolytes and aqueous electrolyte solutions, which conduct electricity due to ion mobility.
Ohm's law is applicable on metallic conductors, it also applies to electrolytic conductors.
According to Ohm’s law, the resistance of a conductor is directly proportional to its length and inversely proportional to its area of cross-section, i.e.,
R ∝ l/a or R = ⍴ x (l/a);
Here, 'l' denotes the length and 'a' denotes the cross-sectional area of the solution column held between the electrodes, and 'R' denotes the solution's resistivity or specific resistance.
Conductivity of Solutions
It is easier to think of conductance (G) than resistance when looking for solutions.
The link between conductance (G) and resistance (R) is as follows:
Conductance = 1 / Resistance or G = (1/R)The ohm-1 or mho is unit of conductance (G). siemens (S) is also the unit of conductance.
As a result, 1 ohm-1 = 1 S.
G = (1/⍴) x (a/l) = 𝜅 x (a/l);
where 𝜅 is called the solution's conductivity or specific conductance.This results in
𝜅 = G x (1/a) = (1/R) x (l/a)Conductivity (𝜅) = (l/a)/R = Cell Constant/Resistance
S m-1 (siemen /metre) is the SI unit for conductivity (𝜅).
Note that 1 S m-1 = 1 ohm-1 m-1.
Equivalent Conductivity
An electrolyte's equivalent conductivity (Λeq) in solution is defined as
“Equivalent conductivity refers to the ability of one equivalent of an electrolyte in a solution to conduct all of the ions it produces.”As a result, equivalent conductivity is written as
Equivalent Conductivity (Λeq) = Conductivity (𝜅)/ Concentration in equivalents per unit volume (Ceq).∴ Λeq = 𝜅/Ceq
Molar Conductivity (ΛM)
The molar conductivity (ΛM) of a solution can also be used to define its conducting power.
Molar conductivity (ΛM) is defined as follows:
“The molar conductivity of any solution is defined as the total conducting power of all the ions supplied by one mole of an electrolyte.”Thus, molar conductivity is expressed as
Molar conductivity (ΛM) = Conductivity (𝜅)/ Concentration in moles per unit volume (Ceq) = 𝜅/Ceq.
Relationship Between Molar and Equivalent Conductivities
The definition says that Λm = 𝜅/Cm, and Λeq = 𝜅/Ceq.
By combining the two equations,
Cm/Ceq = Λeq/Λm.Then, based on equation,
Λeq/Λm = 1/z; where z = 1,2,3,..
Kohlrausch's Law
Kohlrausch’s Law asserts that the conductivity of an electrolytic solution is equal to the sum of the conductivities of both ions at infinite dilution (which are present in the electrolyte).
λ∞eq = λ∞c + λ∞aHere, λ∞eq = Equivalent conductivity at infinite dilution;
λ∞c = cation conductivity, λ∞a = anion conductivity.
Electrolysis
Electrolysis is a chemical degradation of the electrolyte that occurs when an electric current is passed across it.
It takes place in a cell known as an electrolytic cell.
The electrical energy in this cell is converted into chemical energy.
The following elements influence the electrolysis product:
The electrolyte's composition.
The electrode's nature.
Ion concentration in a solution.
Flowing current amount.
Faraday’s Laws of Electrolysis
The link between the amount of electricity transmitted through an electrolyte and the amount of material freed or deposited at the electrode was established by Faraday.
First Law of Electrolysis
The amount of any substance deposited or dissolved at a particular electrode is proportional to the quantity of electricity utilised.
Therefore, from the above definition, w∝Q or w∝(I x t) or w = ZIt.;
where w is the mass of the substance deposited or liberated in grammes, Q is the quantity of charge used in coulombs, I is the current intensity in amperes, t is the time in seconds that current passes through the cell, and Z is the electrochemical equivalent.
Second Law of Electrolysis
The quantity of the deposit is directly proportional to its equivalent weight when the same amount of power is transferred through different electrolytes. (Electrolyte equivalent weight).
Therefore, W/E = constant = F; F= 96500 C per mole = Faraday constant.
Read more about Faraday’s Laws of Electrolysis from Vedantu’s website.
Electrochemical Cell
The cells in which chemical energy is converted to electrical energy are known as electrochemical cells. This means that chemical processes result in the generation of electric current.
The Daniel cell is the most basic electrochemical cell to examine.
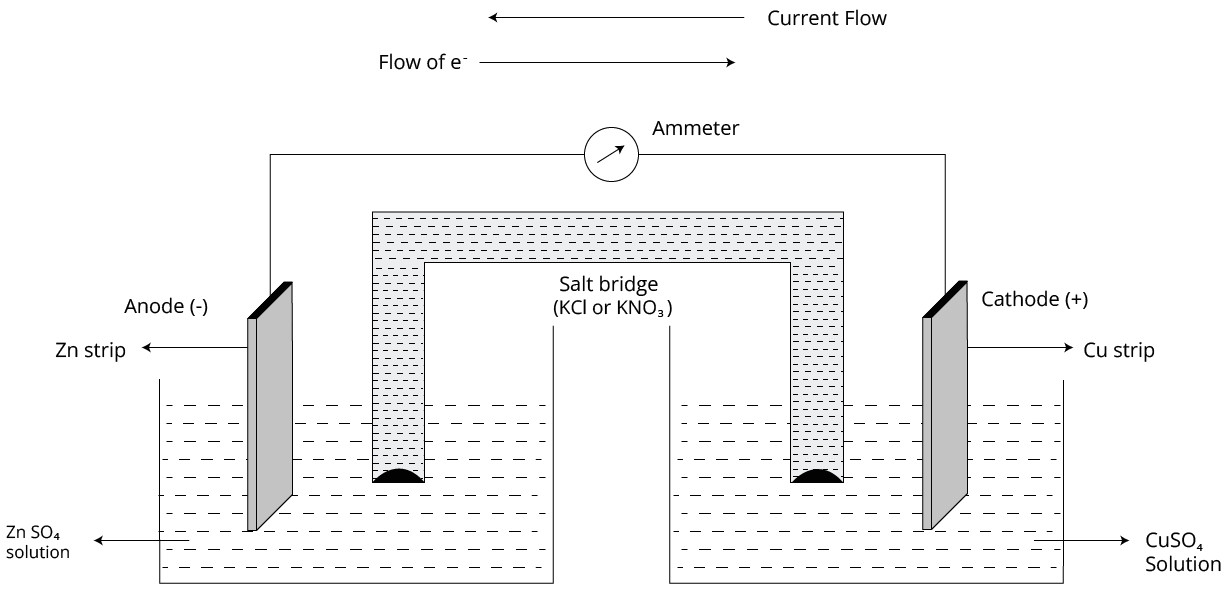
The reactions that take place at the two electrodes are as follows:
At anode: Zn(s) → Zn2+(aq) + 2e-
At cathode: Cu2+(aq) + 2e- → Cu(s)
Electrode Potential
For charge separation, the equilibrium between the metal and its ions is exploited, which results in a potential being formed between the metallic strip and its solution.
The potential difference at equilibrium is determined by the net charge separation:
Metal's and one's own nature.
The temperature and ion concentration in the solution.
EMF of the Cell
Electromotive force (EMF) or cell voltage refers to the difference in electrode potential between the two electrodes of a cell.
EMF = Ered(cathode) – Ered(Anode) or simply as EMF = Ecation – Eanion
Cell Potential and Nernst Equation
The Nernst equation is used to connect a cell's half-cell potential or EMF to the concentration of the species involved.
Consider the case of a redox change in an electrochemical cell: xA + yB ⇌ zC + aD;
where A, B, C, and D are the species with varying concentrations, i.e., gases or solution phases.Ecell = E°cell = 0.059/n * $log\dfrac{[C]^{z}[D]^{a}}{[A]^{x}[B]^{y}}$.
∴ Ecell = E°cell = 0.059/n * log($\left[Product\right]/\left[Reactant\right]$).
These equations are known as the Nernst equation, and they apply to both half-cell and complete-cell reactions.
Relation Between Gibbs Free Energy and EMF
Gibbs free energy can be calculated by multiplying the total charge driven through the cell by the potential difference.
Thus, -ΔG = Total charge x EMF of the cell
-ΔG = nF x Ecell
The negative sign denotes a decrease in free energy, implying that as the cell gets more positive, the G will become more negative, resulting in a spontaneous reaction.
Similarly, -ΔG° = nFE°cell.
You can read more about Electrochemistry from Vedantu’s website.
What are the Applications of Redox Reactions?
The versatile Application of Redox Reactions extends across technology, environment, and industry. Crucial in batteries, corrosion protection, and water treatment, these reactions shape advancements in energy storage and environmental cleanup. Understanding the diverse Application of Redox Reactions is pivotal in navigating complex challenges and driving innovations across scientific disciplines.
Here are some of the major application of Redox Reactions:
Batteries: Redox reactions are the foundation of battery technology, converting chemical energy into electrical energy. Common examples include alkaline batteries and lithium-ion batteries.
Corrosion Protection: Corrosion involves redox reactions. Coating metals with substances like zinc or chromium forms a protective layer, preventing oxidation and corrosion.
Metallurgy: Redox reactions are central to extracting metals from their ores. For instance, in the extraction of iron from iron ore (Fe2O3), the reduction of iron oxide to iron involves a redox reaction.
Fuel Cells: These devices convert chemical energy directly into electrical energy through redox reactions. Hydrogen fuel cells, for instance, involve the oxidation of hydrogen and reduction of oxygen.
Chemical Synthesis: Redox reactions play a key role in organic and inorganic synthesis. Oxidation and reduction reactions are often used to produce specific compounds.
Respiration: Cellular respiration in living organisms involves a series of redox reactions, where glucose is oxidized to produce energy in the form of ATP.
Water Treatment: Redox reactions are employed in water treatment processes, such as the removal of pollutants and disinfection.
Photography: The development of photographs relies on redox reactions. Silver halide crystals are reduced to form the visible image during photographic processing.
Environmental Cleanup: Redox reactions are used to remediate polluted environments. For example, microbial processes can facilitate the reduction of contaminants.
Electroplating: Redox reactions are utilized in electroplating to coat one metal with a thin layer of another, enhancing its appearance or corrosion resistance.
What is Redox Titration?
Redox titration is a chemical analysis technique used to determine the concentration of a substance in a sample by measuring the amount of electrons gained or lost during a redox reaction. The process involves a titrant (a solution of known concentration) reacting with the analyte (the substance being analyzed) to establish the equivalence point. Indicators or electrodes can then be used to detect the endpoint, allowing for precise concentration calculations. Common examples include the determination of the concentration of reducing agents like Fe2+ or oxidizing agents like KMnO4 in a solution.
What are the Application of Redox Titration?
In the realm of analytical chemistry, the Application of Redox Titration emerges as a cornerstone, offering precise insights into diverse substances. Its robust methodology finds Application of Redox Titration in quantifying vitamins, assessing water quality, and ensuring pharmaceutical and food product integrity. With Applications of Redox Titration spanning environmental analysis and industrial quality control, this technique stands as an indispensable tool for accurate concentration determinations across various scientific domains.
Redox titrations find widespread applications in various fields due to their precision and versatility. Some notable applications include:
Determination of Vitamin C Content:
Redox titrations are employed to measure the vitamin C (ascorbic acid) content in food and pharmaceuticals, as it is sensitive to oxidation.
Quantification of Iron in Foods:
The concentration of iron in food products is determined using redox titrations. This is crucial for assessing nutritional content.
Water and Wastewater Analysis:
Redox titrations are utilized to measure the oxygen demand in water samples, providing insights into water quality and environmental impact.
Analysis of Disinfectants:
The concentration of disinfectants like chlorine in water treatment processes can be determined through redox titrations.
Quality Control in Pharmaceuticals:
Redox titrations are applied to assess the concentration of certain pharmaceutical ingredients, ensuring product quality and adherence to standards.
Analysis of Electroplating Baths:
Redox titrations help monitor the concentration of metal ions in electroplating solutions, ensuring the quality of plated materials.
Quantification of Peroxides:
Redox titrations are employed to measure the concentration of peroxides, which can be crucial in various industrial processes.
Analysis of Bleaching Agents:
Redox titrations are used to determine the concentration of bleaching agents in products such as laundry bleach.
Wine and Food Industry:
Redox titrations are utilized in the analysis of wine, determining the concentration of substances like sulfites.
Determination of Copper in Brass:
Redox titrations help quantify the copper content in brass and other alloys, ensuring compliance with industry standards.
What are Redox Reaction Examples?
Redox (reduction-oxidation) reactions involve the transfer of electrons between reactants, resulting in changes to their oxidation states. In the context of JEE Main studies, comprehending redox reactions is pivotal, given their ubiquitous presence in chemistry.
Combustion: Burning fuels like methane $(CH_4)$ or hydrogen $(H_2)$ involve redox reactions with oxygen $(O_2)$ from the atmosphere. For instance, the combustion of methane can be represented as $(CH_4 + 2O_2 \rightarrow CO_2 + 2H_2O)$ .
Corrosion: Oxidation of metals like iron due to exposure to oxygen and moisture leads to rust formation. The reaction of iron with oxygen to form iron oxide $(Fe_2O_3)$ is a classic example of redox.
Bleaching: The process of bleaching involves the reduction of colored compounds. For instance, in the bleaching of cloth using sodium hypochlorite $(NaOCl)$, the hypochlorite ion acts as an oxidizing agent.
Battery Operation: Batteries operate through redox reactions. For example, in a zinc-carbon battery, oxidation of zinc $(Zn)$ at the anode releases electrons used in the reduction of manganese dioxide $(MnO_2)$ at the cathode.
Understanding these examples is crucial for JEE Main aspirants as redox reactions constitute fundamental principles in chemistry. Mastery of these reactions aids in comprehending more complex chemical processes and applications, laying a strong foundation for further studies in the field.
JEE Main Redox Reactions and Electrochemistry Solved Examples
Question 1: In the following, identify the oxidising and reducing agents:
(a) 2H2(g) + O2(g) → 2H2O(g)
(b) Cu(s) + 4HNO3(aq) → Cu(NO3)2(aq) + 2NO2(g) + 2H2O(l)
Solution:
Calculate the oxidation numbers and compare them.
An increase in the oxidation number represents oxidation.
A drop in the oxidation number represents reduction.
(a) 2H2(g) + O2(g) → 2H2O(g)
H2 was oxidised (Oxidation Number of H: 0 → +1); H2 is the reducing agent.
O2 was reduced (Oxidation Number of O: 0 → -2); O2 is the oxidising agent.(b) Cu + 4HNO3 → Cu(NO3)2 + 2NO2 + 2H2O
Cu has been oxidised (Oxidation Number of Cu: 0 → +2), and it is the reducing agent.
The oxidising agent HNO3 was reduced (Oxidation Number of N: +5 → +4).
Key Points to Remember: Accurate calculation of the changes in the oxidation and reduction number after having identified the oxidising agent and the reducing agent respectively, is the most important concept while solving these types of problems.
Question 2: Balance the following equations using the oxidation number method:
Al(s) + H2SO4(aq) → Al2(SO4)3(aq) + H2(g)
Solution:
Step 1: Give each element an oxidation number.
In Al(s), Al → 0;
In H2SO4(aq), H → +1, S → -6; O → +1;
In Al2(SO4)3(aq), Al → +3, S → -6; O → +1;
In H2, H → 0.
Step 2: Determine which species are oxidised and which are reduced.
From the above, it is clear that Al is oxidised and Hydrogen gets reduced.Step 3: Determine e-gained and e-lost.
Al - 3e-→ Al3+;
2H+ + 2e- → H2.Step 4: To make e- lost equal to e- acquired, multiply by factors and apply the factors as coefficients.
Step 5: Complete the balancing process by inspecting the results.
Hence, the final answer is: 2Al(s) + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2(g).
Key Points to Remember: Accurate calculation of the changes in the oxidation and reduction number after having identified the oxidising agent and the reducing agent, respectively, is the most important concept while solving these types of problems. It might so happen that for certain chemical species the oxidation state will remain unchanged. Care should be taken while handling such chemical species.
Solved Examples of Previous Year Question Papers
Question 1: Given : XNa2HAsO3 +YNaBrO3+ZHCl → NaBr + H3AsO4 + NaCl
The values of X, Y and Z in the above redox reaction are respectively :
(1) 2, 1, 3
(2) 3, 1, 6
(3) 2, 1, 2
(4) 3, 1, 4
Solution:
The equation for a balanced equation is shown below:
3Na2HAsO3 + NaBrO3 + 6HCl → NaBr + 3H3AsO4 + 6NaCl
X, Y, and Z have the values 3, 1, and 6 correspondingly.
As a result, option (2) is the correct answer.
Question 2: Consider the reaction
H2SO3(aq) + Sn4+(aq) + H2O(l) → Sn2+(aq) + HSO4–(aq) + 3H+(aq)
Which of the following statements is correct?
(1) H2SO3 is the reducing agent because it undergoes oxidation
(2) H2SO3 is the reducing agent because it undergoes reduction
(3) Sn4+ is the reducing agent because it undergoes oxidation
(4) Sn4+ is the oxidising agent because it undergoes oxidation
Solution:
The loss of electrons by a molecule during a reaction is referred to as oxidation.
Because it undergoes oxidation, H2SO3 is the reducing agent in the above equation.
As a result, option 1 is the correct answer.
Question 3: In which of the following reactions H2O2 acts as a reducing agent ?
(1) H2O2 + 2H+ + 2e– → 2H2O
(2) H2O2 - 2e– → O2 + 2H+
(3) H2O2 + 2e– → 2OH–
(4) H2O2 + 2OH– - 2e– → O2 + 2H2O
(1) (1), (3)
(2) (2), (4)
(3) (1), (2)
(4) (3), (4)
Solution:
In a redox chemical process, a reducing agent is an element or molecule that loses an electron to an electron recipient.
H2O2 functions as a reducing agent in (2) and (4).
As a result, option (2) is the correct answer.
Practice Questions
Question 1: In each of the following reactions, identify the species that is being oxidised and reduced:
(a) Cr+ + Sn4+ → Cr3+ + Sn2+
(b) 3Hg2+ + 2Fe(s) → 3Hg2 + 2 Fe3+
(c) 2As(s) + 3Cl2(g) → 2AsCl3
Answer:
(a) Cr+: oxidised, Sn4+: reduced.
(b) Hg2+: reduced, Fe: oxidised.
(c) As: oxidised, Cl2: reduced.
Question 2: Write balanced equations for the following redox reactions:
(a) NaBr + Cl2 → NaCl + Br2
(b) Fe2O3 + CO → Fe + CO2 in acidic solution
(c) CO + I2O5 → CO2 + I2 in basic solution
Answer:
(a) 2NaBr + Cl2 → 2NaCl + Br2
(b) Fe2O3 + 3CO → 2Fe + 3CO2 in acidic solution.
(c) 5CO + I2O5 → 5 CO2 + I2 in basic solution.
JEE Main Chemistry Redox Reactions and Electrochemistry Study Materials
Here, you'll find a comprehensive collection of study resources for Redox Reactions and Electrochemistry designed to help you excel in your JEE Main preparation. These materials cover various topics, providing you with a range of valuable content to support your studies. Simply click on the links below to access the study materials of Redox Reactions and Electrochemistry and enhance your preparation for this challenging exam.
JEE Main Chemistry Study and Practice Materials
Explore an array of resources in the JEE Main Chemistry Study and Practice Materials section. Our practice materials offer a wide variety of questions, comprehensive solutions, and a realistic test experience to elevate your preparation for the JEE Main exam. These tools are indispensable for self-assessment, boosting confidence, and refining problem-solving abilities, guaranteeing your readiness for the test. Explore the links below to enrich your Chemistry preparation.
Conclusion
The JEE chapter on Redox Reactions and Electrochemistry holds paramount significance in the world of chemistry and its applications. It unravels the complex interplay of electrons in chemical reactions, emphasizing the pivotal concepts of oxidation, reduction, and the assignment of oxidation states. Understanding redox reactions is not only a fundamental aspect of chemical knowledge but also integral to solving intricate problems in analytical chemistry, electrochemical processes, and industrial applications. The comprehension of electrochemical cells, Faraday's laws, and electrode potentials equips students with the tools to delve into diverse fields, from energy storage to corrosion prevention. Mastering this chapter is indispensable for JEE aspirants, offering profound insights into the electrifying world of chemical transformations and electrochemistry.
Redox Reactions and Electrochemistry Chapter - Chemistry JEE Main

 Share
ShareFAQs on Redox Reactions and Electrochemistry Chapter - Chemistry JEE Main
1. What are the key rules for assigning oxidation states in complex species like peroxides, superoxides, and metal carbonyls that frequently appear in JEE Main?
For JEE Main, it's crucial to know the exceptions to standard oxidation state rules:
- Peroxides (e.g., H₂O₂, Na₂O₂): Oxygen has an oxidation state of -1.
- Superoxides (e.g., KO₂, RbO₂): Oxygen has an oxidation state of -1/2.
- Oxides with Fluorine (e.g., OF₂): Oxygen has an oxidation state of +2, as fluorine is more electronegative.
- Metal Carbonyls (e.g., Ni(CO)₄): The carbonyl ligand (CO) is neutral, so the central metal atom has an oxidation state of zero.
2. For JEE Main problems, when is it more efficient to use the ion-electron method versus the oxidation number method for balancing redox reactions?
The choice of method depends on the reaction type:
- The ion-electron (half-reaction) method is generally more reliable and systematic for reactions in aqueous ionic media (acidic or basic), as it clearly separates the oxidation and reduction processes.
- The oxidation number method can be faster for molecular equations or when the reaction does not explicitly occur in an aqueous medium. It focuses on tracking the change in oxidation numbers to balance the electron transfer directly.
For complex reactions in JEE, the ion-electron method is often preferred to avoid errors.
3. How are Faraday's laws of electrolysis applied to solve quantitative problems in JEE Main involving mass deposited and current?
Faraday's laws are used for stoichiometric calculations in electrolysis:
- Faraday's First Law (w = ZIt): This relates the mass (w) of a substance deposited at an electrode to the current (I) and time (t). The electrochemical equivalent (Z) is calculated as E/F, where E is the equivalent weight and F is the Faraday constant (approx. 96500 C/mol).
- Faraday's Second Law (w₁/E₁ = w₂/E₂): This law is used when the same quantity of electricity is passed through different electrolytes. It states that the mass deposited is directly proportional to the equivalent weight of the substance.
These laws are fundamental for solving problems on coulombs passed, moles of electrons, and mass of products formed.
4. What is the Nernst equation and how does it help calculate cell potential under non-standard conditions for JEE Main 2026?
The Nernst equation is a critical formula in electrochemistry that relates the cell potential (E_cell) under non-standard conditions to the standard cell potential (E°_cell) and the concentrations of reactants and products. The equation is:
E_cell = E°_cell - (2.303RT / nF) log₁₀Q
At 298 K (25°C), this simplifies to:
E_cell = E°_cell - (0.0591 / n) log₁₀Q
Here, 'n' is the number of moles of electrons transferred in the balanced redox reaction, and 'Q' is the reaction quotient. It is essential for problems where concentrations or pressures are not at standard state (1M or 1 atm).
5. How does dilution affect specific, molar, and equivalent conductivity for both strong and weak electrolytes?
The effect of dilution on conductivity is a key concept:
- Specific Conductivity (κ): For both strong and weak electrolytes, specific conductivity decreases upon dilution. This is because the number of current-carrying ions per unit volume of the solution decreases.
- Molar (Λ_m) and Equivalent (Λ_eq) Conductivity: For both types of electrolytes, molar and equivalent conductivity increase upon dilution. For strong electrolytes, the increase is gradual as inter-ionic attraction decreases. For weak electrolytes, the increase is sharp because dilution increases the degree of dissociation (α), significantly raising the total number of ions in the solution.
6. How does the Nernst equation connect the concepts of Gibbs Free Energy (ΔG) and the equilibrium constant (K)?
The Nernst equation provides a powerful link between three fundamental thermodynamic and electrochemical concepts. The relationships are:
- ΔG° = -nFE°_cell: Connects standard Gibbs free energy change to standard cell potential, indicating spontaneity.
- ΔG° = -RTlnK: Connects standard Gibbs free energy change to the equilibrium constant.
By equating these two expressions for ΔG°, we get nFE°_cell = RTlnK, or E°_cell = (RT/nF)lnK. This shows that a positive E°_cell implies K > 1 and a spontaneous reaction, directly linking cell potential to the position of equilibrium.
7. Why is a salt bridge essential in a galvanic cell, and what are the ideal characteristics of its electrolyte?
A salt bridge serves two critical functions in a galvanic cell:
- It completes the electrical circuit by allowing the migration of ions between the two half-cells, which prevents the accumulation of charge.
- It maintains the electrical neutrality of the solutions in both half-cells. Anions from the salt bridge move to the anode compartment, and cations move to the cathode compartment.
The electrolyte in a salt bridge (e.g., KCl, KNO₃, NH₄NO₃) must be chemically inert and contain ions that have similar ionic mobilities to ensure a balanced flow of charge.
8. What is a disproportionation reaction, and how can standard electrode potentials (E°) be used to predict if it will occur?
A disproportionation reaction is a specific type of redox reaction where a single species in an intermediate oxidation state is simultaneously oxidised and reduced to form two different products. For example, the decomposition of hydrogen peroxide (H₂O₂) into water (H₂O) and oxygen (O₂). A species will undergo disproportionation if the standard electrode potential for the overall reaction is positive. This condition is met if the E° for the reduction of the species to a lower oxidation state is greater than the E° for its oxidation to a higher oxidation state.




















 Watch Video
Watch Video


