




How to Identify sp, sp2 and sp3 Hybridisation in Molecules
The concept of Hybridisation is fundamental in JEE Main Chemistry as it explains how atomic orbitals merge to form new, directionally optimised hybrid orbitals. This mixing governs molecular shape, bond angles, and reactivity patterns—making Hybridisation a core principle for understanding organic and inorganic bonding mechanisms. Mastering the types of hybridisation (sp, sp2, sp3) and knowing how to quickly assign the correct hybridisation to any atom is essential for fast problem-solving and avoiding exam traps. Hybridisation also helps justify why molecules like methane (CH4), ethene (C2H4), or CO2 have their characteristic geometries. Vedantu summarizes this high-yield topic for JEE aspirants at an NCERT and competitive level.
What is Hybridisation?
Hybridisation in chemistry is the process where atomic orbitals (usually s, p, and sometimes d orbitals of the same atom) mix to form new, equivalent hybrid orbitals. These hybrid orbitals are better oriented for strong, stable chemical bonding and help explain the observed geometry of molecules. For example, sp3 hybridisation in carbon explains the tetrahedral shape of methane.
Key Features and Importance of Hybridisation
- The number of hybrid orbitals formed equals the number of atomic orbitals mixed.
- Hybrid orbitals are equivalent in shape and energy, improving directional bonding.
- Hybridisation rationalises molecular geometry and bond angles in both organic and inorganic compounds.
- Essential for understanding chemical bonding and molecular structure questions in JEE exams.
Types of Hybridisation: sp, sp2, sp3 and Beyond
Different types of hybridisation arise depending on how many and which orbitals are mixed. JEE Main usually focuses on sp, sp2, and sp3 types, but sp3d and sp3d2 are also tested for inorganic compounds.
| Type | Orbitals Involved | Geometry | Bond Angle | Examples |
|---|---|---|---|---|
| sp | 1 s + 1 p | Linear | 180° | CO2, C2H2 |
| sp2 | 1 s + 2 p | Trigonal planar | 120° | BF3, C2H4 |
| sp3 | 1 s + 3 p | Tetrahedral | 109.5° | CH4, NH3, H2O |
| sp3d | 1 s + 3 p + 1 d | Trigonal bipyramidal | 90°, 120° | PCl5, SF4 |
| sp3d2 | 1 s + 3 p + 2 d | Octahedral | 90° | SF6, [CoF6]3− |
These variations allow for a vast range of molecular shapes critical in both organic frameworks and coordination compounds. For diagrams and advanced cases like sp3d, see detailed Vedantu notes.
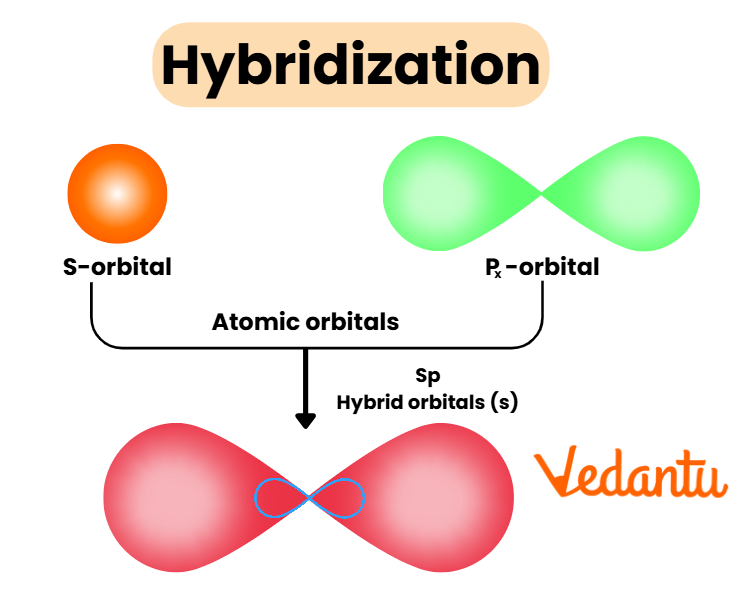
Rules and Steps: How to Identify Hybridisation Quickly
- Calculate the number of sigma bonds (any single bond, or one per double/triple bond).
- Add the number of lone pairs on the atom being considered.
- Total (sigma bonds + lone pairs) = number of hybrid orbitals required.
- Assign: 2 → sp; 3 → sp2; 4 → sp3; 5 → sp3d; 6 → sp3d2.
Example: The central atom in NH3 has 3 sigma bonds with hydrogen and 1 lone pair, so it uses 4 hybrid orbitals—thus, sp3 hybridisation.
Illustration: Hybridisation in JEE Main Molecule Examples
- Methane (CH4): Carbon forms 4 sigma bonds—sp3 (tetrahedral, 109.5°).
- Ethene (C2H4): Each C atom forms 3 sigma bonds—sp2 (trigonal planar, 120°), unhybridised p forms the π bond.
- Acetylene (C2H2): Each C forms 2 sigma bonds—sp (linear, 180°), 2 unhybridised p for 2 π bonds.
- Boron trifluoride (BF3): B makes 3 sigma bonds—sp2 (planar).
- PCl5: 5 sigma bonds—sp3d (trigonal bipyramidal geometry).
- SF6: 6 sigma bonds—sp3d2 (octahedral).
Shapes from Hybridisation: Molecular Geometry Prediction
The shape of a molecule is dictated by its hybridisation type and the VSEPR theory. Recognising these shapes allows speedy MCQ elimination in JEE papers:
- sp – Linear molecule: Example CO2
- sp2 – Trigonal planar: Example BF3
- sp3 – Tetrahedral: Example CH4
- sp3d – Trigonal bipyramidal: Example PCl5
- sp3d2 – Octahedral: Example SF6
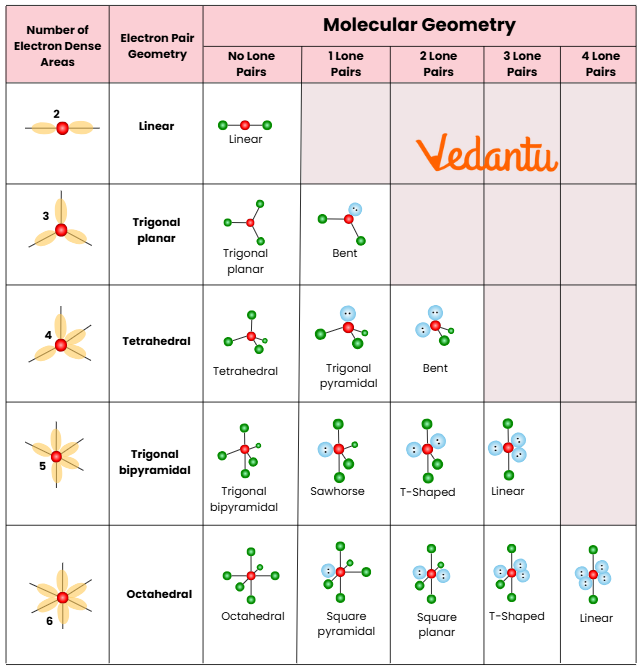
Hybridisation vs Hybridisation in Biology: Key Difference
| Chemistry Hybridisation | Biology Hybridisation |
|---|---|
| Mixing of atomic orbitals of one atom to form hybrid orbitals for bonding | Crossbreeding of two different plants/animals for improved offspring |
| Determines molecule shape and bond angle | Determines genetic traits in next generation |
| Central to valence bond theory and molecular models | Central to genetics and selective breeding |
Be careful not to confuse molecular hybridisation (chemistry) with genetic hybridisation (biology) in interdisciplinary questions.
Common Mistakes and JEE Quick Tips
- Lone pairs must be counted when determining the number of regions of electron density (avoid overlooking them for geometry).
- π bonds are NOT included in the count of hybrid orbitals (only sigma bonds + lone pairs).
- Some compounds with expanded octets (e.g. SF6, PCl5) require inclusion of d orbitals for hybridisation—check group number and period.
- Bond angle deviations happen due to lone pair repulsion or double bond character; ideal bond angles apply only for perfectly symmetrical cases.
- Learn common exceptions: NH3 (sp3—pyramidal) and H2O (sp3—bent) are frequently tested in MCQs.
- Revise using hybridisation examples for carbon, and compare with questions from BF3 hybridisation.
Fast Revision Table for Hybridisation: JEE Main Essentials
| Hybridisation | Arrangement | Ideal Bond Angle | Examples |
|---|---|---|---|
| sp | Linear | 180° | CO2, BeCl2 |
| sp2 | Trigonal Planar | 120° | BF3, C2H4 |
| sp3 | Tetrahedral | 109.5° | CH4, NH3, H2O |
Use this table as a rapid check before attempting JEE Main chemical bonding questions or during quick revision.
Applications: Why Hybridisation Matters for JEE Mains
- Explains the structure and reactivity of common organic compounds, such as alkanes, alkenes, alkynes.
- Central to mechanisms in organic chemistry involving resonance and intermediate stability.
- Predicts molecular geometry and helps identify correct VSEPR shapes (see NH3, CO2, BCl3 practice).
- Helpful in topics like coordination compounds and molecular orbital theory.
- Essential while solving advanced practice papers and previous year questions.
Practice and Next Steps: Mastering Hybridisation for JEE
Regularly practice assigning hybridisation to atoms in diverse molecules and ions. Review solved examples, MCQs, and diagrams from Vedantu’s expert notes, and ensure you understand the distinction between sigma and pi bonds, lone pairs, and formal charge considerations. For a deeper dive, explore:
- Chemical Bonding Mock Test
- Hybridisation of CH4
- BF3 Hybridisation Detailed
- In-depth Hybridisation Notes
- Hybridisation of SF4
- Hybridisation of XeF4
- Organic Compounds Containing Halogens
- H2O Hybridisation Case Study
For concise, authentic notes and exam-level questions on Hybridisation and related topics, Vedantu is trusted by top JEE educators nationwide. Consistent practice and fast recall of Hybridisation rules will give you confidence in JEE Main Chemistry MCQs and reasoning.
FAQs on Hybridisation in Chemistry – Concept, Types & Applications
1. What do you mean by hybridisation in chemistry?
Hybridisation in chemistry is the process where atomic orbitals mix to form new, equivalent hybrid orbitals, explaining molecular shape and bonding.
- It combines s, p (and sometimes d) orbitals to produce hybrid orbitals.
- These hybrid orbitals help predict molecular geometry like linear, trigonal planar, or tetrahedral.
- The concept is crucial for understanding organic compounds and their properties in JEE/NEET exams.
2. How can I identify if a molecule has sp, sp2, or sp3 hybridisation?
You can identify the type of hybridisation by counting the total number of electron domains (bond pairs + lone pairs) around the central atom.
- sp hybridisation: 2 electron domains ⇒ linear (180°)
- sp2 hybridisation: 3 electron domains ⇒ trigonal planar (120°)
- sp3 hybridisation: 4 electron domains ⇒ tetrahedral (109.5°)
- Use the formula: Number of hybrid orbitals = Number of sigma bonds + lone pairs on central atom.
3. What is the difference between hybridisation in biology and chemistry?
Hybridisation in chemistry refers to the mixing of atomic orbitals for bonding, while in biology it means crossing two different species or varieties.
- Chemistry: Orbital mixing to explain molecular geometry.
- Biology: Breeding technique to produce hybrids with desirable traits.
- Both processes create new combinations, but in totally different fields and contexts.
4. Why is hybridisation important in organic chemistry?
Hybridisation explains the structure, shape, and bonding in organic molecules like alkanes, alkenes, and alkynes.
- Helps understand bond angles and reactivity.
- Essential for predicting properties of carbon compounds.
- Used to solve JEE Main and NEET questions on molecular structure.
5. Which types of questions are asked on hybridisation in JEE Main?
JEE Main frequently asks both theory and numerical questions on hybridisation:
- Determining the hybridisation type of given atoms/molecules
- Calculating or identifying bond angles and molecular shapes
- Hybridisation in organic reactions and mechanisms
- Drawing Lewis structures and predicting geometry.
- Recognising exceptions or special cases in hybridisation.
6. How to determine hybridisation of a central atom quickly in a molecule?
To quickly determine hybridisation, count the number of sigma bonds and lone pairs on the central atom:
- 2 regions: sp
- 3 regions: sp2
- 4 regions: sp3
- Formula: Hybridisation = number of sigma bonds + lone pairs
- Practice with common molecules (e.g., CH4, BF3, BeCl2) for speed.
7. Can a single atom have hybrid orbitals of different types at the same time?
Typically, a single atom adopts one type of hybridisation in a particular molecule, but in complex or excited states, different types may coexist for different bonds.
- For example, Carbon in ethene (C2H4) uses sp2 for its geometry.
- In transition states or resonance structures, atoms may show mixed character.
- However, in most exam questions, only one dominant type is considered per atom.
8. Is hybridisation always required to explain bonding in all molecules?
Hybridisation explains bonding in many molecules, especially organics, but some molecules are better explained by molecular orbital theory.
- Useful for predicting shapes and bond angles in main-group compounds.
- Some exceptions or special cases (like O2, NO) require alternative models.
- For main exam syllabus, focus on where hybridisation applies as per NCERT/JEE/NEET curriculum.
9. Do d-orbitals participate in hybridisation for main-group elements?
d-orbitals usually participate in hybridisation for elements in or beyond the 3rd period of the periodic table (e.g., P, S, Cl).
- Examples: PF5 (sp3d), SF6 (sp3d2)
- For 2nd period elements (like C, N, O, F), d-orbitals are not available for hybridisation.
10. Are bond angles always ideal for a given hybridisation type?
Bond angles for a given hybridisation are often ideal (e.g., 109.5° for sp3), but can be slightly less due to lone pair repulsion or multiple bonds.
- Lone pairs reduce bond angles (e.g., NH3 has 107° instead of 109.5°).
- Double/triple bonds can also affect angles.
- Always check for exceptions based on lone pairs or π-bonds.

































