




What is Excess Reagent?
During a chemical reaction, one of the reactants is found to be present in a higher quantity than what is necessary for the completion of the reaction. This reactant, which is present in greater quantity, is not utilised completely and is always left behind even after the completion of the reaction. It remains even after the chemical equilibrium is reached. This is the excess reactant or excess reagent definition.
On the other hand, there are some other reactants that get fully consumed during the course of the reaction. At the end of the reaction, all of it has been reacted completely and none of it is left behind. Such reactants are referred to as the limiting reagent. The amount of the limiting reagent is what decides the quantity of the product formed.
Let us consider the equation,
$2 \mathrm{~N}_{2}+3 \mathrm{H}_{2} \rightarrow 2 \mathrm{NH}_{3}+\mathrm{N}_{2}$
It represents the reaction of nitrogen with hydrogen. This balanced reaction equation shows that 2 N2 atoms react with 3 H2 atoms. This indicates that if there is more than 2 N2, it will remain unreacted. We say that nitrogen is present in excess of hydrogen . Therefore, nitrogen is the excess reagent whereas hydrogen is the limiting reagent.
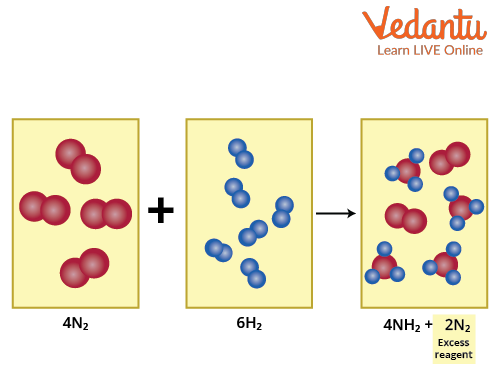
Formation of Ammonia showing Nitrogen is the Excess Reagent
Method of Determining Excess Reagent Along With Examples
Let us first understand the concept using a general and non-chemistry example.
Consider the manufacturing of a bicycle. All of us have ridden a bicycle at some point in our lives. We know it has 2 wheels and a seat. Now imagine you have 50 wheels and 30 seats. Of these two (reactants), which one of them is in excess can be determined by calculating the number of bicycles (products) formed in the end. This is done using the unitary method.
2 wheels $\rightarrow$ 1 bicycle
$\text { Hence for } 50 \text { wheels } \rightarrow \dfrac{50 \text { wheels }}{2 \text { wheels }} \times 1 \text { bicycle }=25 \text { bicycles }$
Similarly,
1 seat $\rightarrow$ 1 bicycle
Hence for $30 \text { seats } \rightarrow \dfrac{30 \text { seats }}{1 \text { seat }} \times 1 \text { bicycle }=30 \text { bicycles }$
Since there are only 50 wheels that can be used to manufacture 25 bicycles, even if there are 30 seats, there is no use because only 25 bicycles can be made. Therefore, in this example, the wheels act as limiting reagents and decide the number of end products formed, whereas the seats act as excess reagents since they are in greater quantity than what is required for the manufacturing process.
Example 1. Now let us understand the same concept in a stepwise manner by taking up a chemistry example.
Consider the combustion of methane,
$\mathrm{CH}_{4}+\mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+\mathrm{H}_{2} \mathrm{O}$
Always make sure the chemical equation is balanced in order to determine the quantity of excess reagent.
$\mathrm{CH}_{4}+2 \mathrm{O}_{2} \rightarrow \mathrm{CO}_{2}+2 \mathrm{H}_{2} \mathrm{O}$
To find the excess reagents, we need to know their molar masses.
The molar mass of CH4 = 12 + (4$\times$1) = 16 grams per mole
The molar mass of O2 = 2 × (16$\times$2) = 64 grams per mole
This indicates 64 grams of oxygen are utilised for the combustion of 16 grams of methane.
Now let us assume we have only 8 grams of methane and 50 grams of oxygen available for the reaction to occur. Just like how we calculate the wheels and seats used for manufacturing bicycles, we use the unitary method to determine the amount of oxygen used for the combustion of 8 grams of methane.
For combustion of $16 \text { grams of } \mathrm{CH}_{4} \rightarrow 64 \text { grams of } \mathrm{O}_{2}$ is consumed
Hence for combustion of $8 \text { grams of } \mathrm{CH}_{4} \rightarrow \dfrac{64 \text { grams }}{16 \text { grams }} \times 8 \text { grams }=32 \text { grams of } \mathrm{O}_{2}$ is required
We found out that we only need 32 grams of O2 for the combustion of oxygen. But, we have 50 grams present in the reaction. Therefore, it is present in more quantity than what is necessary for the combustion of methane. Therefore, oxygen is an excess reactant or excess reagent.
Amount of excess oxygen = 50 - 32 = 18 grams.
While CH4 is the limiting reagent, the reaction stops after 8 grams of methane is used up.
Example 2: Consider the reaction equation of the formation of sodium chloride,
$\mathrm{Na}+\mathrm{Cl}_{2} \rightarrow \mathrm{NaCl}$
Balance the chemical equation,
$2 \mathrm{Na}+\mathrm{Cl}_{2} \rightarrow 2 \mathrm{NaCl}$
To find the excess reagents, we need to know their molar masses.
The molar mass of Na = 23 grams per mole
molar mass of Cl2 = 2 $\times$ 35.5 = 71 grams per mole
This indicates 23 grams of sodium will react with 71 grams of chlorine.
Now let us assume we have 46 grams of sodium and 230 grams of chlorine available for the reaction to occur.
Now, we will apply the unitary method.
23 grams of $\mathrm{Na}$ reacts with $\rightarrow 71$ grams of $\mathrm{Cl}_{2}$
Hence 46 grams of $\mathrm{Na}$ would react with $\rightarrow \dfrac{71 \text { grams }}{23 \text { garms }} \times 46$ grams $=142$ grams of $\mathrm{Cl}_{2}$
We found out that only 142 grams of Cl2 is needed for the reaction with Na. But, we have 230 grams present in the reaction. Therefore, it is present in more quantity than what is necessary for the reaction. Therefore, chlorine is an excess reactant or excess reagent.
The amount of excess chlorine = 230 - 142 = 88 grams.
While sodium is the limiting reagent, the reaction stops after 46 grams of sodium has reacted.
Difference Between Excess Reagent and Limiting Reagent
Summary
An excess reactant is the one that is not completely consumed during the reaction. It is present in more quantities than necessary. It has no impact on the quantity of the end product formed. It is the reactant that remains after the chemical reaction has stopped.
On the other hand, a limiting reagent is the one that is used up completely during a chemical reaction. It is the first one to be utilised completely. It determines the amount of product formed. None of the reagents is left at the end of the reaction.
FAQs on Excess Reagents - Important Topic for JEE
1. What is a marginal reagent? Given an example.
A marginal reagent is nothing but a limiting reagent itself. It is completely used up during the chemical reaction and determines when the reaction comes to an end. None of it is left at the end of the reaction. It is the main factor that determines the quantity of the product formed.
Example: $2 \mathrm{AgI}+\mathrm{Na}_{2} \mathrm{~S} \rightarrow \mathrm{Ag}_{2} \mathrm{~S}+2 \mathrm{NaI}$
From the above balanced chemical equation, the ratio between silver iodide and sodium sulphide is 2:1. If 1 mole of each reactant is used for the reaction, silver iodide will be consumed entirely, but sodium sulphide will still remain in excess. Therefore, silver iodide is the limiting or marginal reagent.
2. Can there be more than one limiting reagent?
Having more than one limiting reactant in a chemical reaction is not possible. This is because one reactant acting as a limiting reagent has already been used up. This means that the reaction cannot reach completion or the reaction does not proceed any further. As a result, a reaction will have only one limiting reagent and one excess reactant. Once the limiting reagent has been utilized, even if there is excess reagent left out, no further product is formed, thereby limiting the reaction.
























