




Difference between SN1 and SN2 reaction: A Detailed Comparison
Nucleophilic substitution reactions play a crucial role in organic chemistry, with two prominent types: SN1 and SN2 reactions. Both mechanisms involve the substitution of a leaving group with a nucleophile, but they differ significantly in their processes, rates, and the factors that influence them. The SN1 reaction occurs in two distinct steps, beginning with the formation of a carbocation intermediate, while the SN2 reaction is a single, concerted step where the nucleophile attacks the substrate simultaneously as the leaving group departs. This article delves into these two types of nucleophilic substitutions, highlighting their key differences, the conditions under which they occur, and their practical applications in the real world.
SN1 Vs SN2: Mechanisms and Key Differences
Here are the illustrations of the SN1 and SN2 reactions mechanism:
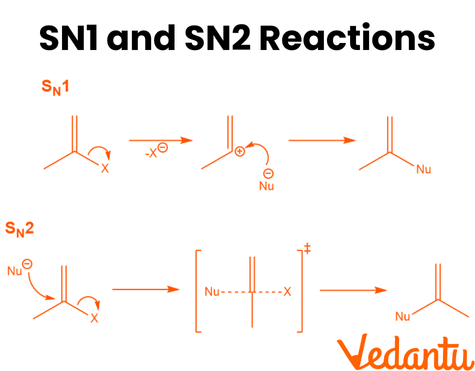
Detailed Mechanisms of SN1 and SN2 Reactions
SN1 Reaction Mechanism:
Step 1: Departure of the Leaving Group
The reaction begins when the leaving group departs from the substrate, forming a carbocation. This step is slow and is the rate-determining step of the reaction.
Step 2: Nucleophilic Attack
Once the carbocation is formed, the nucleophile attacks the positively charged carbon atom, leading to the substitution product. The nucleophile can attack from either side, leading to possible racemization (inversion and retention of configuration).
Key Factors:
Substrate Structure: Tertiary carbocations are most stable, so the SN1 mechanism is favored with tertiary alkyl halides.
Solvent: Polar protic solvents stabilize the carbocation intermediate, aiding the reaction.
Nucleophile: The nucleophile can be weak, as its role is secondary to the carbocation formation.
SN2 Reaction Mechanism:
One-Step Mechanism:
In the SN2 mechanism, the nucleophile attacks the substrate at the same time as the leaving group departs. This occurs in a single concerted step, with no intermediates formed.
Key Features:
Stereochemistry: The nucleophile attacks the substrate from the opposite side of the leaving group, resulting in a complete inversion of the configuration at the carbon atom (known as Walden inversion).
Substrate Structure: The reaction is fastest with methyl and primary substrates, as these structures have less steric hindrance for the nucleophile to attack.
Solvent: Polar aprotic solvents, like DMSO or acetone, are preferred because they do not solvate the nucleophile, allowing it to remain reactive.
Real-World Applications of SN1 and SN2 Reactions
SN1 Reactions: SN1 reactions are commonly used in the synthesis of tertiary alkyl halides, where a stable carbocation can form. For example, SN1 mechanisms are involved in the formation of alcohols from alkyl halides. The use of SN1 reactions is also widespread in pharmaceuticals, where specific stereochemistry is required for drug activity.
SN2 Reactions: SN2 reactions are widely employed in organic synthesis, especially for converting primary alkyl halides to various functional groups. In industrial processes, SN2 reactions are used for synthesizing nucleophilic substitution compounds, such as in the creation of esters or ethers. Due to the inversion of configuration, SN2 reactions are often used in cases where a specific stereochemical outcome is required.
Key Factors Affecting SN1 and SN2 Reactions
Substrate Structure:
SN1: Tertiary > Secondary > Primary
SN2: Methyl > Primary > Secondary > Tertiary (less favorable due to steric hindrance)
Solvent:
SN1: Polar protic solvents (e.g., water, alcohols) favor carbocation formation.
SN2: Polar aprotic solvents (e.g., DMSO, acetone) help the nucleophile remain unencumbered.
Nucleophile:
SN1: Weak nucleophiles can still participate due to the slow formation of the carbocation.
SN2: Strong nucleophiles are necessary for effective attack.
Conclusion
The difference between SN1 and SN2 reactions lies primarily in their mechanisms, reaction conditions, and factors that affect the rate and outcome. Understanding these differences is crucial for predicting reaction paths and choosing the appropriate conditions for specific organic reactions. Whether it's for synthesizing new compounds or studying reaction kinetics, knowing when to apply SN1 or SN2 reactions can significantly influence the success of chemical processes.
FAQs on Understanding the Mechanisms and Key Differences in SN1 and SN2 Reactions
1. What are the primary differences between SN1 and SN2 reactions for JEE Advanced?
The primary differences between SN1 and SN2 reactions lie in their mechanism, kinetics, and stereochemical outcomes. For JEE Advanced, a clear understanding is crucial:
- Mechanism: The SN1 reaction is a two-step process involving the formation of a stable carbocation intermediate. The SN2 reaction is a single, concerted step where the nucleophile attacks as the leaving group departs.
- Rate Law: The SN1 reaction rate is unimolecular, Rate = k[Substrate], as it only depends on the substrate concentration. The SN2 reaction rate is bimolecular, Rate = k[Substrate][Nucleophile].
- Substrate Preference: SN1 is favoured by tertiary (3°) and secondary (2°) substrates that form stable carbocations. SN2 is favoured by methyl and primary (1°) substrates due to minimal steric hindrance.
- Stereochemistry: SN1 reactions typically result in racemization (a mix of inversion and retention) due to the planar carbocation intermediate. SN2 reactions result in a complete inversion of configuration (Walden Inversion).
2. How can one predict if a nucleophilic substitution will follow an SN1 or SN2 pathway?
To predict the reaction pathway, analyse the following factors in order:
- Substrate Structure: This is the most important factor. If the substrate is tertiary, the reaction is almost always SN1. If it is primary or methyl, it is almost always SN2. If it is secondary, other factors become decisive.
- Nucleophile Strength: A strong nucleophile (e.g., OH⁻, CN⁻) favours the SN2 pathway because it can effectively attack the substrate in the concerted step. A weak nucleophile (e.g., H₂O, ROH) favours the SN1 pathway as it waits for the carbocation to form.
- Solvent Type: Polar protic solvents (like water or ethanol) stabilize the carbocation intermediate, strongly favouring SN1. Polar aprotic solvents (like acetone or DMSO) do not solvate the nucleophile well, making it more reactive and favouring SN2.
- Leaving Group: A better leaving group (e.g., I⁻ > Br⁻ > Cl⁻) increases the rate of both SN1 and SN2 reactions.
3. Why do SN1 reactions lead to racemization while SN2 reactions cause a complete inversion of stereochemistry?
This difference is a direct consequence of their mechanisms. In an SN1 reaction, the rate-determining step is the departure of the leaving group, forming a planar, sp²-hybridized carbocation intermediate. This flat structure allows the incoming nucleophile to attack from either the top or bottom face with nearly equal probability, leading to a mixture of enantiomers known as a racemic mixture.
In contrast, an SN2 reaction involves a single, concerted step. The nucleophile performs a backside attack on the carbon atom, approaching from the side opposite the leaving group. This forces the other three groups to 'flip' over, similar to an umbrella inverting in the wind. This process, known as Walden Inversion, results in a product with a configuration that is completely inverted relative to the reactant.
4. How does the choice of solvent critically influence the competition between SN1 and SN2 mechanisms?
The solvent plays a crucial role by stabilising or destabilising the intermediates and reactants involved:
- Polar Protic Solvents (e.g., water, methanol): These solvents have acidic protons that can form hydrogen bonds. They are excellent at solvating both the cation (carbocation intermediate) and the anion (leaving group). This strong stabilisation of the carbocation intermediate significantly lowers the activation energy for the first step of the SN1 reaction, making it the preferred pathway.
- Polar Aprotic Solvents (e.g., acetone, DMSO, DMF): These solvents have dipoles but lack acidic protons. They can solvate cations but are poor at solvating anions (the nucleophile). This leaves the nucleophile 'naked' and highly reactive, increasing its ability to attack the substrate directly. This condition strongly favours the bimolecular collision required for the SN2 reaction.
5. Why is steric hindrance a deciding factor for the SN2 mechanism but less critical for the SN1 mechanism?
Steric hindrance is crucial for the SN2 mechanism because of its transition state. In the SN2 transition state, the carbon atom is simultaneously bonded to the incoming nucleophile and the outgoing leaving group, resulting in a crowded, five-coordinate arrangement. Bulky groups on or near the carbon atom physically block the nucleophile's path for a backside attack, drastically slowing down or preventing the reaction.
For the SN1 mechanism, the rate-determining step is the formation of the carbocation. This step actually relieves steric strain as the geometry changes from a crowded tetrahedral (sp³) to a less crowded trigonal planar (sp²) structure. The subsequent attack by the nucleophile on the planar carbocation is not sterically hindered, making initial steric bulk a non-issue for the reaction rate.
6. What happens with secondary alkyl halides? Can they undergo both SN1 and SN2 reactions?
Yes, secondary (2°) alkyl halides are a borderline case and can undergo both SN1 and SN2 reactions. The actual pathway depends on a competition governed by the reaction conditions. The outcome is determined by the strength of the nucleophile and the type of solvent:
- To favour SN2: Use a strong, non-bulky nucleophile (like CN⁻ or I⁻) in a polar aprotic solvent (like acetone). These conditions promote the bimolecular attack before the carbocation has a chance to form.
- To favour SN1: Use a weak nucleophile (like H₂O or CH₃OH) in a polar protic solvent. These conditions favour the formation of a secondary carbocation intermediate. It's important to note that for secondary substrates, SN1 reactions are often in competition with E1 elimination reactions.
7. What is the difference in the rate laws for SN1 and SN2 reactions, and what does it imply about their mechanisms?
The rate laws provide direct evidence for the number of molecules involved in the rate-determining step of a reaction.
- SN1 Rate Law: Rate = k[Substrate]. This is a first-order rate law, meaning the rate depends only on the concentration of the alkyl halide (the substrate). This implies that the slowest step of the reaction involves only the substrate molecule, which corresponds to the formation of the carbocation. The nucleophile is not involved in this step.
- SN2 Rate Law: Rate = k[Substrate][Nucleophile]. This is a second-order rate law, meaning the rate depends on the concentrations of both the substrate and the nucleophile. This implies that the slowest step involves a collision between both of these species, which supports the model of a single, concerted step where the nucleophile attacks as the leaving group departs.
8. In the context of JEE Advanced, what role does the leaving group play in both SN1 and SN2 reactions?
The ability of the leaving group to depart is critical for both SN1 and SN2 reactions, as it is involved in the rate-determining step of both pathways. A good leaving group is a species that is a weak base and stable on its own after detaching. The general trend for halide leaving groups is I⁻ > Br⁻ > Cl⁻ > F⁻.
- In SN1 reactions: A better leaving group accelerates the reaction by lowering the activation energy for carbocation formation.
- In SN2 reactions: A better leaving group also accelerates the reaction by making the carbon atom more susceptible to nucleophilic attack and by stabilising the transition state.
Therefore, a substrate with a good leaving group will react faster regardless of whether the mechanism is SN1 or SN2.























