




Why Is Iodine Important? Everyday Uses and Scientific Insights
Iodine has many industrial uses. For example, it is used in the production of polyvinyl chloride and plastics such as polystyrene and polyvinylidene chloride. It is also used to make rubber products such as elastic bands, clothes, shoes, and tires.
Iodine can be found in the human body. The thyroid gland needs iodine to make thyroid hormones that regulate how your body uses energy from food (metabolism). Iodine deficiency can lead to a goitre which can cause problems with breathing or swallowing food.
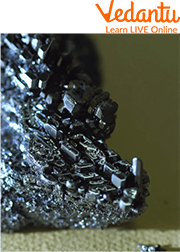
Iodine
What is Iodine?
Iodine is an element belonging to the halogens. It is non-metal, meaning it has no electrons in its outer shell, and as such can bind with other elements much more easily. The nucleus of iodine is composed of four particles: two neutrons, one proton, and one electron. It was discovered in 1811 by French chemist Bernard Courtois, who was born in 1778, but named it after the Greek word for violet because of its colour.
Iodine has a melting point of 254 oC and boiling point of 314 oC at normal atmospheric pressure. Although it is heavier than water, it is not toxic enough to be considered a heavy metal.
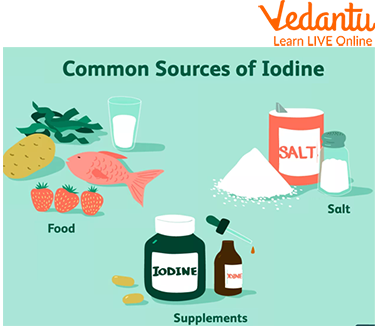
Common Sources of Iodine
Uses of Iodine

Rich Sources of Iodine
Iodine is a chemical element that has many uses in the world, including medical and industrial purposes. Iodine's most important uses are:
Iodine is important for thyroid hormone production.
Iodine helps regulate metabolism and maintain cell growth.
Deficiency can lead to hypothyroidism, goitre, and mental retardation.
Iodine is found in saltwater fish such as cod, salmon, and shrimp. It can also be found in dairy products like milk or yoghurt, as well as bread containing iodised salt.
What does Isotope of Iodine Treat?
The isotope of iodine is used to treat a variety of disorders in the treatment of cancer. It is given to patients before and during radiation therapy, to increase the absorption of radioactive iodine into the thyroid gland. It is also given to patients after total thyroidectomy (removal) and/or placement of radioactive iodine seeds placed in the neck under local anaesthesia because it helps destroy residual thyroid tissue, which can lead to the recurrence of the disease.
Iodine Ointment Uses
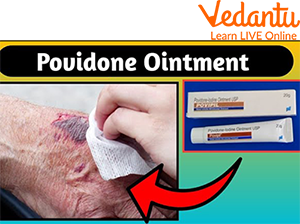
Iodine Ointment
Iodine ointment is typically used to treat skin conditions like eczema, psoriasis and acne. It has been in use since 1835 and helps treat a wide variety of skin problems by slowing down the growth of bacteria on the skin. It can help soften dry, scaly or cracked skin and ease itching as well.
It can also be used to relieve pain from insect bites or stings, allergic reactions, sunburns or other burns. The use of iodine ointment on an infant's umbilical cord can help prevent skin at the base of the cord from becoming infected.
Industrial Uses of Iodine

Industrial Use of Iodine
Iodine is an essential micronutrient that has many uses in industries. Major applications of iodine rank from the production of iodised salt to the creation of polyvinylpyrrolidone (PVP) and alkyl iodides. Iodine is a nonmetallic, toxic, and volatile element with low reactivity. PVP is polymerized into fibres that are used in water purification membranes because iodine ions can separate out hydrogen ions from water molecules to create hydrogen gas.
Summary
In this article, we learned that iodine is a chemical element discovered by French chemist Bernard Courtois in 1811, and he named it after the Greek goddess of the same name. It is an essential trace mineral for human health. We also learned the uses of iodine in everyday life range from its use as a food additive to its use as a treatment for thyroid disorders. Iodine is an important part of our daily lives and it can be found in many things such as table salt, bread, milk, seafood, and seaweed.
Iodine is a chemical element essential for life and essential to the human diet. However, iodine deficiency can lead to thyroid problems. It also has other roles in the body, such as regulating metabolism and maintaining cell growth.
FAQs on Uses of Iodine: Essential Concepts for Students
1. What are the most common uses of iodine?
Iodine has several important uses across different fields. The primary applications include:
- Medical Use: It is widely used as an antiseptic to clean wounds and sterilise skin before surgery in the form of tincture of iodine or povidone-iodine.
- Dietary Supplement: Iodine is an essential nutrient for humans. It is added to table salt (iodised salt) to prevent iodine deficiency disorders.
- Diagnostic Tool: Radioactive isotopes of iodine are used in medical imaging to monitor thyroid gland function.
- Chemical Industry: It serves as a catalyst in the production of acetic acid and synthetic rubber.
- Laboratory Reagent: In school and college labs, the iodine test is a standard method to check for the presence of starch.
2. What are the main dietary sources of iodine?
The best way to get iodine is through diet. The most significant sources of iodine include:
- Iodised Salt: The most common and reliable source for most people.
- Seafood: Fish like cod and tuna, as well as shrimp and other shellfish, are naturally rich in iodine.
- Seaweed: Kelp, nori, and kombu are some of the most concentrated natural sources of iodine.
- Dairy Products: Milk, cheese, and yoghurt contain iodine.
- Eggs: The yolk of an egg is a good source of this essential mineral.
3. How is iodine used as an antiseptic, for example, in an iodine tincture?
Iodine is a powerful antiseptic because it can effectively kill a wide range of microorganisms, including bacteria, viruses, and fungi. An iodine tincture is a solution of iodine dissolved in alcohol and water. When applied to a cut or wound, it penetrates the cell walls of germs and disrupts their proteins and nucleic acids, leading to their death. This helps in preventing infection and promoting safe healing. Povidone-iodine is a more modern, less irritating formulation commonly used in hospitals.
4. What is iodine and what are its key chemical properties?
Iodine is a chemical element with the symbol I and atomic number 53. It belongs to the halogen group on the periodic table. Key properties include:
- State: At room temperature, it is a lustrous, greyish-black crystalline solid.
- Sublimation: A unique property of iodine is that it undergoes sublimation, meaning it turns directly from a solid into a violet-coloured gas when heated, bypassing the liquid state.
- Chemical Formula: As a molecule, it exists as I₂.
- Solubility: It is only slightly soluble in water but dissolves well in organic solvents and aqueous solutions of potassium iodide.
5. What happens to the human body due to iodine deficiency?
Iodine deficiency can lead to several health problems, collectively known as Iodine Deficiency Disorders (IDD). The most common effect is on the thyroid gland, which enlarges in an attempt to capture more iodine from the blood, causing a swelling in the neck called a goitre. Other consequences include hypothyroidism (underactive thyroid), leading to fatigue, weight gain, and depression. In children, severe deficiency can cause stunted growth and impaired cognitive development, a condition known as cretinism.
6. Why is iodine essential for thyroid function and hormone production?
Iodine is the fundamental building block for thyroid hormones. The thyroid gland absorbs iodine from the bloodstream and incorporates it into two crucial hormones: thyroxine (T4) and triiodothyronine (T3). These hormones are the primary regulators of the body's metabolism, controlling how quickly the body uses energy. They are vital for brain development, heart function, muscle control, and maintaining bone health. Without sufficient iodine, the thyroid cannot produce these hormones, leading to a slowdown of all bodily functions.
7. How is the 'iodine test' used in biology to detect the presence of starch?
The iodine test is a simple and effective chemical test used to identify the presence of starch. When an iodine solution (typically Lugol's iodine, which is iodine-potassium iodide) is added to a substance containing starch, a chemical reaction occurs. The iodine molecules slip inside the helical structure of the starch molecule, forming a complex that absorbs light differently. This results in a distinct colour change, turning the yellowish-brown iodine solution into a deep blue-black colour. This test is commonly used in biology labs to confirm photosynthesis in leaves by testing them for starch.
8. Are there any risks or side effects from consuming too much iodine?
Yes, while iodine is essential, excessive intake can be harmful and lead to iodine toxicity. Consuming too much iodine can paradoxically cause some of the same symptoms as deficiency, such as goitre and hypothyroidism. It can also lead to hyperthyroidism (overactive thyroid), where the gland produces too much hormone. Symptoms of toxicity can include a burning sensation in the mouth, fever, nausea, and in severe cases, it can cause thyroid gland inflammation. It is important to consume iodine within the recommended dietary allowances.
9. Besides its medical and dietary roles, what are some industrial uses of iodine?
Beyond its well-known health applications, iodine has several important industrial uses. Some key examples include:
- LCD Screens: Iodine is used to make polarising films, which are essential components of Liquid Crystal Displays (LCDs) found in TVs, computers, and smartphones.
- Chemical Synthesis: It acts as a catalyst in various industrial chemical processes, such as the production of acetic acid and certain types of synthetic rubber.
- Animal Feed: Iodine is often added as a supplement to livestock feed to ensure proper growth and health in animals.
- Water Purification: Iodine tablets can be used to disinfect water for drinking in emergency situations.
10. How does iodised salt help prevent diseases like goitre on a large scale?
Iodised salt is one of the most successful public health initiatives worldwide. The strategy works because salt is an inexpensive staple food item used consistently by nearly all populations, regardless of economic status. By fortifying salt with a small, safe amount of potassium iodate or iodide, it becomes a simple vehicle to deliver this essential micronutrient to everyone. This ensures that the population's baseline iodine needs are met, effectively preventing Iodine Deficiency Disorders (IDD) like goitre and cretinism on a massive scale without requiring people to change their dietary habits.









