




Properties, Uses, and Environmental Impact of Mercury
Have you ever wondered what the silver liquid inside the thermometer is? Well, it is an element called Mercury. Quicksilver is another name for the liquid, silvery-shiny metal mercury. With the atomic weight of Mercury being 200.59 and the atomic number of mercury 80 on the periodic chart, it is a transition metal with the element symbol Hg.
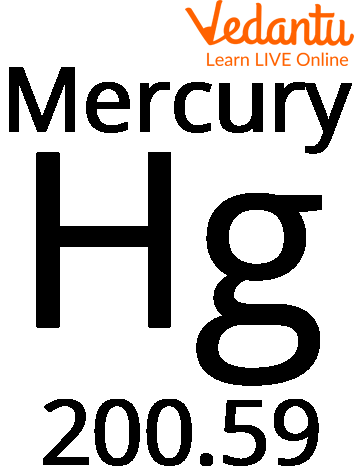
Symbol of Mercury
Who Discovered the Mercury Element?
Mercury has been discovered in Egyptian tombs that date back to around 1500 B.C., and it was known to the ancient Chinese, Egyptians, and Hindus. Mercury is referred to as "hydro-argyros" by Aristotle in his writings from the fourth century B.C., which translates to "liquid silver" or "water silver."

Discovery of Mercury
Is Mercury a Metal?
Historically known as quicksilver, elemental or metallic mercury is a gleaming, silver-white metal that is liquid at ambient temperature.
Where Can You Find Liquid Mercury?
Like other metals, mercury is collected from ores, with cinnabar being one of the most common types. China, Spain, and California are where you can find them most often. The ore does contain liquid mercury in the form of drops, however, it is typically removed through heating.
What is Mercury Used For?
Use of Mercury in Thermometers
There are Various Uses of Mercury Shown Below:
Because of its homogeneous expansion and contraction, elemental mercury is used in thermometers, blood pressure monitors, and thermostats to measure changes in temperature and pressure.
Additionally, fluorescent lighting, paints, soaps, batteries, and dental fillings all contain mercury.
Tin and silver are alloyed with mercury in the amalgam used in dental fillings. Mercury has been employed as a fungicide in paint because it functions as a biocide, however, this type of paint is no longer available.
Adverse Effects of Mercury
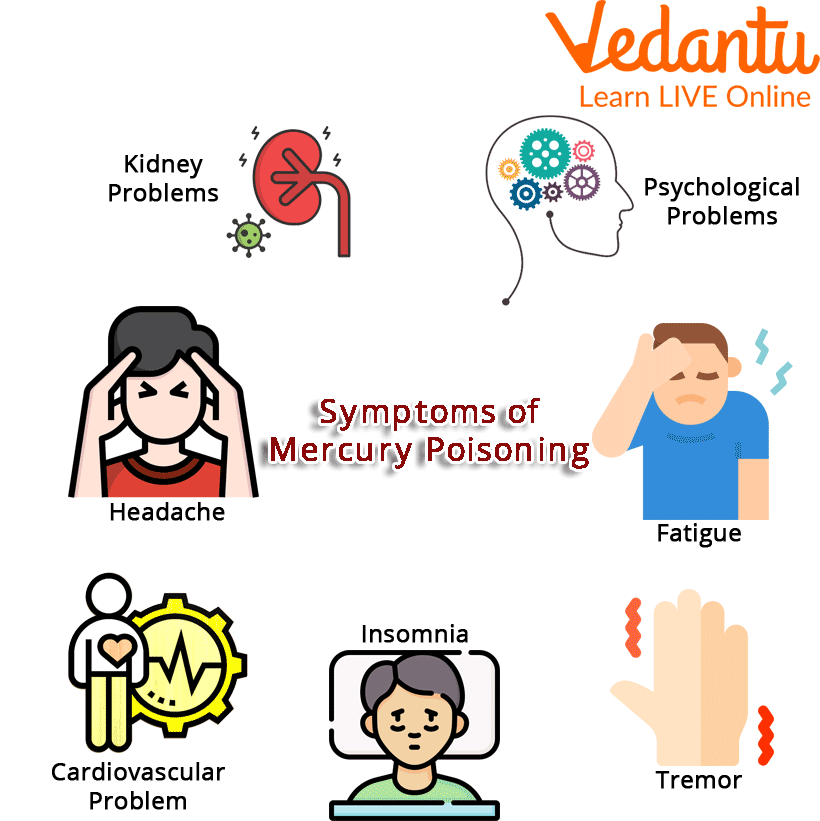
Symptoms of Mercury Poisoning
One of the best ways to help the environment is to stop using certain dangerous substances like mercury, which can be found in some energy-efficient appliances. Mercury can cause serious damage if it gets into water supply systems, creating health risks for humans and animals alike. There is no safe level of mercury exposure - even low levels can be harmful.
Effects of Mercury on the Human Body
The central and peripheral neurological systems are hazardous to both elemental and methylmercury. Mercury vapour inhalation may be lethal and have detrimental effects on the lungs, kidneys, neurological, digestive, and immune systems. The inorganic salts of mercury are harmful to the kidneys if consumed and are corrosive to the skin, eyes, and gastrointestinal tract.
Summary
Thus, here we have discussed the symbol of mercury, the atomic number of mercury, the atomic weight of mercury, where liquid mercury can be found, the effect of mercury and all other important topics related to mercury. The smallest planet in the solar system and one that is nearest to the Sun is Mercury. Mercury vapour inhalation may be fatal and have adverse effects on the lungs, kidneys, neurological, digestive, and immune systems.
FAQs on Is Mercury a Metal? Learn All You Need to Know
1. What is mercury, and is it classified as a metal?
Yes, mercury is classified as a metal. It is a unique chemical element, also known as quicksilver, with the atomic number 80. Despite being a liquid at standard room temperature, it exhibits key metallic properties like being a good conductor of electricity and forming alloys with other metals. It belongs to the transition metals group in the periodic table.
2. Why is mercury a liquid at room temperature when most metals are solid?
Mercury is a liquid at room temperature due to its unique atomic structure and relativistic effects. The electrons in a mercury atom are held very tightly by the nucleus, leading to unusually weak metallic bonds between its atoms. Unlike the strong bonds that hold atoms of metals like iron or gold in a solid lattice, mercury's weak bonds are easily overcome by the thermal energy available at room temperature, allowing its atoms to flow freely as a liquid.
3. What are the main uses of mercury in industry and science?
Mercury has several specific applications due to its unique properties. Some of the main uses include:
Thermometers and Barometers: Its uniform expansion and contraction with temperature made it ideal for these instruments, though this use is now declining due to toxicity concerns.
Electrical Switches and Relays: Liquid mercury can be used to create silent, reliable electrical contacts in certain types of switches.
Fluorescent Lighting: Mercury vapour is essential for the operation of fluorescent and compact fluorescent lamps (CFLs).
Mining: It is used in artisanal and small-scale gold mining to form an amalgam with gold, separating it from ore.
4. Why is mercury considered highly toxic to humans and the environment?
Mercury is highly toxic because it is a potent neurotoxin, meaning it can severely damage the central nervous system, brain, and kidneys. When released into the environment, bacteria can convert it into an even more dangerous organic form called methylmercury. This substance builds up in aquatic organisms (a process called bioaccumulation) and becomes more concentrated up the food chain. Humans are primarily exposed by consuming contaminated fish, which can lead to severe and permanent health problems.
5. What is the chemical symbol for mercury and what is its origin?
The chemical symbol for mercury is Hg. This symbol is an abbreviation of its old Latin name, hydrargyrum, which translates to "water-silver" or "liquid silver." This name was chosen because it perfectly describes mercury's shiny, silver-like appearance and its unusual liquid state at room temperature.
6. How does mercury differ from more common metals like iron or copper?
The most obvious difference is its physical state; mercury is a liquid at room temperature, while iron and copper are strong solids. This is because of mercury's exceptionally weak metallic bonds. Other key differences include:
Density: Mercury is extremely dense (13.5 g/cm³), much denser than iron (7.8 g/cm³) or copper (8.9 g/cm³).
Toxicity: Mercury and its compounds are highly toxic, whereas iron and copper are essential micronutrients for most living organisms in small amounts.
Volatility: Mercury is relatively volatile for a metal and can evaporate to form a toxic vapour, even at room temperature.
7. Where does mercury naturally occur, and how does it get into our environment?
Mercury occurs naturally in the Earth's crust, primarily within the reddish ore called cinnabar (mercuric sulfide). It is released into the environment through natural events like volcanic eruptions and the weathering of rocks. However, a significant amount of mercury pollution comes from human activities, such as:
The burning of coal in power plants.
Industrial processes like manufacturing chlorine and cement.
The improper disposal of mercury-containing products like old thermometers, batteries, and CFL bulbs.
8. What is an amalgam and why is it important in the context of mercury?
An amalgam is a special type of alloy made by mixing mercury with one or more other metals, such as gold, silver, or tin. Mercury has a unique ability to dissolve these metals. This property has been historically important in two main areas:
Dentistry: Dental amalgams, or "silver fillings," are made by mixing mercury with a powder of silver, tin, and copper. This creates a durable material for filling cavities.
Gold Extraction: In small-scale mining, mercury is used to bind with tiny gold particles in ore, forming an amalgam that makes the gold easier to collect.









