




What Is the Ester to Aldehyde Reaction? Mechanism, Uses & Practice
One of the components in synthetic flavours, perfumes and cosmetics is ester. Ester is an organic compound which reacts with water and gives alcohol and acid. RCO2R' is the chemical formula of esters. Aldehydes are another important class of organic compounds which can be used as fungicides, insecticides and germicides in plants and vegetables and also used for the production of polymeric materials. The chemical formula of aldehyde is RCHO. Esters can be reduced to aldehyde.
The ester and aldehyde can also react together. A special type of organic reaction where an alpha halo ester reacts with aldehyde or ketone to give beta hydroxy ester is called the Reformatsky reaction. Beta hydroxy ester has a lot of importance in cosmetics and pharmaceutical industries. In this topic, we are discussing all the details of the Reformatsky reaction, the reaction mechanism and the advantages of the Reformatsky reaction.
What is Reformatsky Reaction?
The Reformatsky reaction was discovered in 1887 by a Russian chemist named Sergey Nikolaevich Reformatsky. The reaction usually takes place between a carbonyl compound and alpha halo ester. The reaction takes place in the presence of zinc metal where inorganic solvents like diethyl ether or THF can be used. This is a condensation reaction and can be adapted to intramolecular aldol condensation. The isolation of organo zinc reagent is not required in a Reformatsky reaction. During the reaction, a new carbon-carbon bond is formed along with the formation of a zinc halide.
Definition of Reformatsky Reaction
Reformatsky reaction is a condensation reaction. Reformatsky reaction can be defined as a reaction between aldehyde or ketones with alpha halo ester and beta hydroxy ester by using zinc metal. Here, zinc helps to produce the organo zinc reagent called Reformatsky enolate. Reformatsky enolate is less reactive compared to the Grignard reagent. Hence, there is no possibility for a nucleophilic addition to the ester group. The reaction is an extended reaction between two carbonyl compounds in the presence of zinc. The solvent used in the reaction is diethyl ether or tetrahydrofuran.
Reaction Mechanism
Formation of zinc enolate: By oxidative addition, zinc metal is inserted to the carbon halogen bond of alpha halo ester.
This compound undergoes dimerisation and rearrangement and forms two zinc enolates.
The oxygen on the aldehyde or ketone coordinates with zinc and a new six-membered transition state is formed.
Zinc moves to the oxygen of aldehyde or ketone and a new carbon-carbon bond is formed.
An acid work up is taken place and zinc is removed by it. Zinc forms Zn(II) salt and we get beta hydroxy ester.
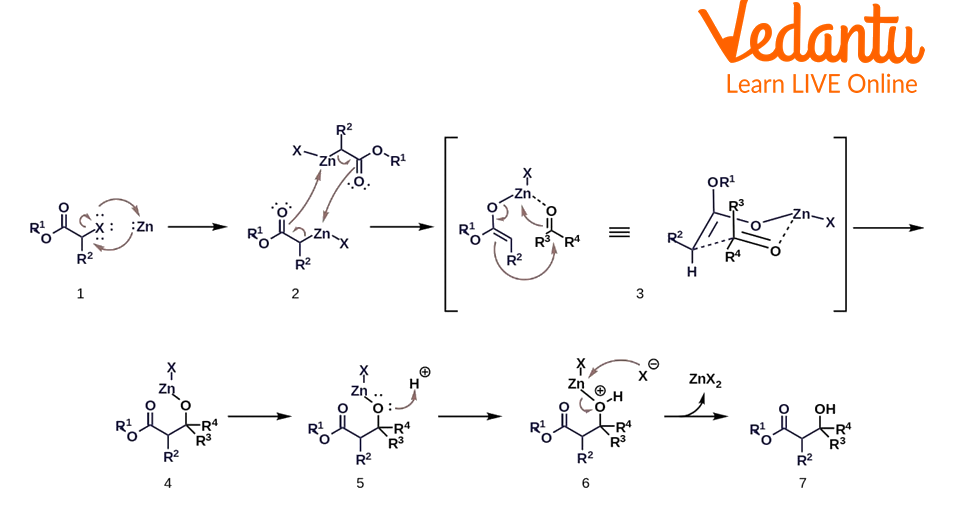
Reformatsky Reaction Mechanism
Advantages of Reformatsky Reaction
Since the Reformatsky enolate is less reactive; hence, the ester group does not undergo nucleophilic addition.
Highly hindered aldehyde or ketones can successfully undergo Reformatsky reaction and nucleophiles can successfully be added to the delta positive carbon of ketones.
The reaction can be adapted to intramolecular aldol condensation very easily.
The organo zinc halide is very stable and it is available in the market.
Reformatsky enolate is an alternative to lithium enolate of ester. Hence, the reaction can be conveniently carried out.
Freshly prepared zinc powder or a heated column of zinc dust can improve the yield of a Reformatsky reaction.
Reformatsky reactions can successfully add carbon nucleophiles to the readily enolizable cyclopentanone ring system.
Uses of Reformatsky Reaction
Reformatsky reaction can be used to produce beta hydroxy ester from alpha halo ester and other carbonyl compounds. The beta hydroxy ester can hydrolysed to produce beta hydroxy acids. Beta hydroxy acids have a large commercial value especially in the cosmetic industry. Beta hydroxy acids can be used in anti-ageing creams and in the pharmaceutical industry.
How Esters can Convert to Aldehyde?
Ester to aldehyde reduction reaction occurs using certain reducing agents. DIBAL-H is the common reducing agent by which ester can be reduced to aldehyde. DIBAL-H is diisobutyl aluminium hydroxide. In order to prevent further reaction of aldehyde, the reaction is carried out at a very low temperature, approximately -780C. Alcohol is a byproduct of this reaction.
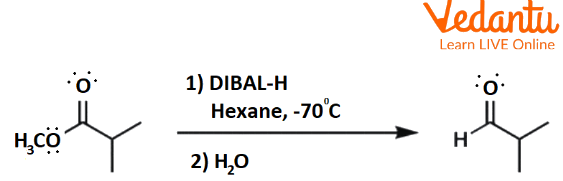
Conversion of Ester to Aldehyde
Interesting Facts
THF complexes of Reformatsky reagents form eight-membered dimers in the solid state.
Various Metals like iron, cobalt, nickel, germanium, cerium, barium, indium, cadmium etc. can be used instead of zinc in the Reformatsky reaction.
Reformatsky reactions of over 500 aldehydes or ketones are identified.
Key Features
Ester can be reduced to aldehyde by using DIBAL-H.
Aldehyde or ketones can react with alpha halo esters and form beta hydroxy esters. This reaction is called the Reformatsky reaction.
Zinc metal is used to generate Reformatsky reagents which are comparatively stable and less reactive than Grignard reagents.
FAQs on Ester to Aldehyde Conversion: Detailed Steps, Examples & Tips
1. What is the primary chemical method used for the conversion of an ester to an aldehyde?
The most common and effective method for the conversion of an ester to an aldehyde is through partial reduction. This is typically achieved using a specific reagent called DIBAL-H (Diisobutylaluminium Hydride) at a very low temperature, usually around -78°C. This controlled reaction prevents the ester from being fully reduced to an alcohol, stopping at the aldehyde stage.
2. Can you explain the mechanism for the reduction of an ester to an aldehyde using DIBAL-H?
The mechanism involves the following key steps:
- Coordination: The Lewis acidic aluminium atom in DIBAL-H coordinates with the carbonyl oxygen of the ester.
- Hydride Transfer: A hydride ion (H⁻) is transferred from the DIBAL-H to the electrophilic carbonyl carbon. This breaks the carbonyl pi bond and forms a stable tetrahedral intermediate.
- Stabilisation: This intermediate is stable at low temperatures (-78°C) and does not collapse.
- Workup: Upon adding water in the workup step, the intermediate hydrolyses to form the final aldehyde product.
3. Why is it crucial to maintain a low temperature (like -78°C) during the reaction with DIBAL-H?
Maintaining a low temperature is critical to prevent over-reduction. The tetrahedral intermediate formed during the reaction is stable only at very low temperatures. If the temperature is allowed to rise, this intermediate would become unstable and collapse, leading to the formation of a primary alcohol instead of the desired aldehyde. The low temperature effectively 'freezes' the reaction at the aldehyde stage before the final aqueous workup.
4. How does the conversion of an ester to an aldehyde differ from its conversion to a primary alcohol?
The key difference lies in the choice of the reducing agent and the extent of reduction.
- Ester to Aldehyde (Partial Reduction): This requires a selective and sterically hindered reducing agent like DIBAL-H at low temperatures. It provides only one hydride ion to stop the reaction at the aldehyde stage.
- Ester to Alcohol (Complete Reduction): This uses a strong, unhindered reducing agent like Lithium Aluminium Hydride (LiAlH₄). LiAlH₄ is much more reactive and will reduce the ester completely to a primary alcohol, without stopping at the intermediate aldehyde stage.
5. Why can't a strong reducing agent like Lithium Aluminium Hydride (LiAlH₄) be used to prepare an aldehyde from an ester?
Lithium Aluminium Hydride (LiAlH₄) is a very powerful and unselective reducing agent. If used with an ester, it would not stop at the aldehyde stage. The initially formed aldehyde is more reactive than the starting ester towards LiAlH₄ and would be immediately reduced further to a primary alcohol. Therefore, LiAlH₄ is used for the complete reduction of esters to alcohols, not for the partial reduction to aldehydes.
6. Can you provide a typical example of an ester to aldehyde conversion?
A classic example is the conversion of Ethyl Ethanoate (an ester) to Ethanal (an aldehyde).
The reaction is written as:
CH₃COOCH₂CH₃ (Ethyl Ethanoate) is treated with:
1. DIBAL-H in a non-polar solvent like toluene at -78°C.
2. H₂O (water) for the workup step.
The final product is CH₃CHO (Ethanal).
























