




An Overview of Class 11 Physics Surface Tension Of Water By Capillary Rise Method Experiment
The phenomenon of surface tension is observed in our daily lives. Surface tension enables small insects to float on water, to disinfect the water using light disinfectants, in the medical diagnosis of jaundice treatment, etc.
It is, therefore, essential to know about the basics of surface tension – its basic idea, principle and theory using the following simple hands-on given regarding the capillary action of water.
Table of Contents
Aim
Setting Up Capillary Tube
Setting Up Reference Needle
Measuring the Height Difference
Result
Aim
To study surface tension of a liquid (water) using the capillary rise method.
Apparatus Required
A Transparent Plastic Capillary Tube
Water
Travelling Microscope
A Beaker
A Cork with Pin
Clamp and Stand
Thermometer
Caustic Soda Solution
A Plumb Line
Theory
Capillary action arises in a liquid due to the mutual attractive forces among the molecules of the liquid and between them and the walls of the container. Capillary action causes the liquid to rise gradually in a fine tube. Liquids having greater surface tension show more prominent capillary action due to stronger intermolecular forces.
When a liquid of density \[\rho \] rises in a capillary tube, the weight of the liquid column below the meniscus is supported by the upward force of surface tension acting around the circumference of the points of contact.
Therefore, if T denotes the surface tension of the liquid, h denotes the height of the liquid column, and r denotes the inner radius of the capillary tube, then
\[2\pi rT = \pi {r^2}h\rho g\] or \[T = \frac{{h\rho gr}}{2}\]
Following is a simple experiment to determine the surface tension of water using capillary rise method. The method uses a travelling microscope to determine the small height of rise of water in a capillary due to surface tension with respect to the surface of water.
Procedure
Clean the capillary tube properly with caustic soda and water.
Fill the beaker with water, measure its temperature.
Clamp the capillary tube vertically using a clamp stand using a plumbline. Move down the tube so that its lower end dips into the water in the beaker.
Surface Tension by capillary rise method
Clamp a cork C above the beaker and push a pin P through it. Lower the cork so that the tip of the pin exactly touches the surface of water (tips of pin and its reflection should coincide).
Wait for 5 minutes. Focus the travelling microscope M on the meniscus of the water level in the capillary tube. Move the microscope until the cross wires in the eye piece are tangential to the lowest point of the meniscus. Note the reading of the travelling microscope.
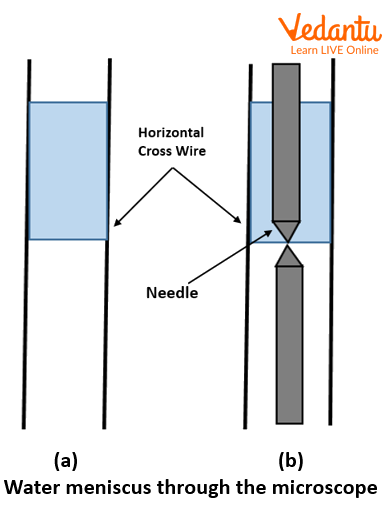
Water meniscus through the microscope
Mark the position of meniscus on the capillary with a pen. Carefully remove capillary tube from beaker without disturbing the pin.
Now focus the microscope on the tip of the pin. Note its reading.
Cut the capillary tube neatly at the marked point. Fix the tube horizontally on a stand. Focus the microscope on the transverse cross section of the tube and take readings of the internal diameter of the tube. Do this for two perpendicular directions.
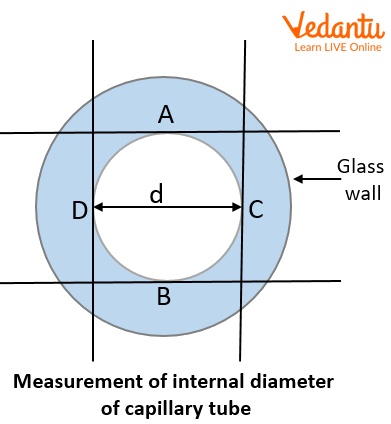
Measurement of internal diameter of capillary tube
Observations
Least (LC) count of the microscope = .......... cm
MSR = Main scale reading
VSD = Vernier scale division
Observation Table – Measurement of Capillary Rise
Mean h = .......... cm
Observation Table – Diameter of Capillary Tube
Mean radius, r = .......... cm
Temperature of water,\[\theta = ..........\;^\circ C\]
Density of water at\[0^\circ C,\;\rho = ..........\;g/c{m^3}\]
Result
From the formula
\[T = \frac{{h\rho gr}}{2}\]
Value of surface tension at ..........\[^\circ C\] is, T = ..........\[N/m\]
Precautions
Capillary tube should be cleaned properly with caustic soda and water.
Tube must be kept vertical while dripping in water.
Temperature should be recorded before and after the experiment.
Height of the liquid column should be measured from the lowest point of the meniscus.
Ensure that the end of the capillary tube is sufficiently dipped into the water.
Lab Manual Questions
1. What would happen if the length of the capillary tube is taken to be less than the height of rise of water?
Ans: When the length of capillary tube is less than the height of rise of water, then the molecules of water upon reaching the top of the tube experience zero surface tension due to horizontal surface. Hence, the liquid ceases to rise any further.
2. What happens to the surface tension of water if soap solution is mixed with water?
Ans: Upon adding the soap solution, the surface tension of water decreases as the effective cohesive force of water molecules decrease.
3. Explain the effect of temperature on surface tension.
Ans: As temperature increases, the kinetic energy of liquid molecules increases which leads to the decrease in the intermolecular force of attraction. Hence, surface tension decreases.
4. How would the phenomena change if the capillary tube gets tilted by a bit?
Ans: If we tilt the capillary tube, the perpendicular height of rise of water from the surface would still be the same as it was before tilting. This is because in a closed tube, the pressure P at the top of the tube is
\[P = {P_0} - \rho gh\]
Where \[{P_0}\] is the atmospheric pressure and h is the height of rise of the liquid.
Viva Questions
1. What is surface tension? Give its definition and explanation.
Ans: Surface tension of a liquid is the tension experienced on the outer surface of the liquid among its particles due to mutual attraction between them. It causes the liquid to form a shape with minimum surface area due to stretching of its surface, much like a film.
2. What is the formula and unit of surface tension?
Ans: The surface tension F is given by the formula
\[T = \frac{F}{{2L}}\]
Where,
F is the force exerted by the liquid
L is the length of the liquid column
The SI unit of surface tension is newton per meter (N/m).
3. What small insects do not get wet when lying on the surface of liquid?
Ans: Surface tension of the liquid acts like a thin stretched film, it binds the surface of the liquid and prevents small bodies such as insects from drowning inside.
4. What are cohesive and adhesive forces?
Ans: The force acting between molecules of the same type is known as cohesive force, whereas that acting between two molecules of different types is known as adhesive force.
5. Enlist the factors affecting surface tension.
Ans: The factors affecting surface tension are:
Nature of the liquid
Nature of the surface in contact
Temperature
6. What is the critical temperature?
Ans: The temperature at which surface tension vanishes is known as critical temperature.
7. Why is the meniscus of water concave but that of mercury is convex?
Ans: The meniscus of water is concave because the adhesive force between water and container molecules is greater than the cohesive force between any two water molecules. On the other hand, the meniscus of mercury is convex because mercury molecules have a stronger cohesive force than adhesive force.
8. What is the angle of contact?
Ans: The angle of contact is defined as the angle subtended between the tangent to the liquid surface drawn at the point of contact and the solid surface inside the liquid.
9. What is the effect of temperature on surface tension?
Ans: As temperature increases, surface tension decreases due to increase in the kinetic energy of liquid molecules.
10. Where does surface tension find practical examples?
Ans: Among practical examples,
A falling drop of liquid assumes a spherical shape.
Usage of soaps and detergents for cleaning.
Rise of oil in the wick of a lamp due to surface tension.
Practical Based Questions
The tension in the free surface of a liquid is called
Stress tension
Strain tension
Surface tension
None of these
Ans: (C) The tension in the free surface of a liquid is called surface tension.
A ball pen works on the principle of:
Viscosity
Boyle’s law
Gravitational force
Surface tension
Ans: (D) A ball pen works on the principle of surface tension.
Among the following, which is not an example of capillary action?
Oil reaching the end of wick
Roots of a plant drawing water from earth
Small insects walking on the surface of water
Water retained in a sponge
Ans: (C) Small insects walking on the surface of water is not an example of capillary action.
What kinds of fluids have zero surface tension?
Real fluids
Ideal fluids
Both A and B
Neither A nor B
Ans: (B) Ideal fluids have zero surface tension.
.......... force is responsible for the spherical shape of rain drops.
Air resistance
Surface tension
Viscosity
Atmospheric pressure
Ans: (B) Surface tension force is responsible for the spherical shape of rain drops
The CGS unit of surface tension is:
Dyne-cm
Dyne/cm
\[Dyne/c{m^2}\]
None of these
Ans: (B) The CGS unit of surface tension is dyne/cm.
Surface tension can be reduced by:
Adding salt
Decreasing temperature
Adding soap solution
All of the above
Ans: (C) Surface tension can be reduced by adding soap solution.
Which among them is the necessary condition for capillary effect?
Adhesive forces must be greater than cohesive forces
Fluid pressure must be zero
Capillary should be porous
Temperature should be very high
Ans: (A) Adhesive forces must be greater than cohesive forces.
Capillary action happens due to:
Surface tension
Density of liquid
Temperature
Length of capillary tube
Ans: (A) Capillary action happens due to surface tension.
The surface tension of a liquid:
Increases with its surface area
Decreases with its surface area
Increases with an increase in temperature
Decreases with an increase in temperature.
Ans: (D) The surface tension of a liquid decreases with an increase in temperature.
Conclusion
From this experiment, we can conclude that the effect of surface tension is prevalent in our surroundings – from lifting the weights of light objects to the cure of many diseases. Surface tension finds many commercial uses such as in the structural design of soaps, detergents, disinfectants, formulation of water compass and capillary action in medicines.
We hope that the reader has got some useful insight of the topic after reading this article and he is motivated to explore the topic in physics for the times to come.
FAQs on Class 11 Physics Surface Tension Of Water By Capillary Rise Method Experiment
1. What is the fundamental principle behind determining the surface tension of water using the capillary rise method for a Class 11 practical exam?
The fundamental principle is that for a liquid that wets the glass, the upward vertical force of surface tension acting along the circumference of contact inside the capillary tube balances the weight of the liquid column that has risen. By measuring the height of this column and the radius of the tube, we can calculate the surface tension.
2. State the formula for surface tension by capillary rise, defining each term. What are the two most crucial measurements for this experiment?
The formula for surface tension (T) as per the CBSE 2025-26 syllabus is: T = (h * ρ * g * r) / 2cosθ. However, for water and clean glass, the angle of contact (θ) is nearly 0, so cosθ ≈ 1, and the simplified formula is T = (hρgr) / 2.
- h = height of the liquid rise in the capillary.
- ρ (rho) = density of the liquid.
- g = acceleration due to gravity.
- r = internal radius of the capillary tube.
The two most crucial measurements are the height (h) and the internal radius (r) of the tube, both measured precisely using a travelling microscope.
3. What are three essential precautions a student must take while performing the capillary rise experiment to get accurate marks?
For an accurate result in this important Class 11 experiment, students must observe the following precautions:
- The capillary tube must be perfectly clean and free from grease to ensure a consistent angle of contact.
- The tube should be set perfectly vertical. Any tilt will lead to an incorrect reading for the length of the water column.
- The height 'h' must be measured from the bottom of the meniscus to the free surface of the water in the beaker.
4. Explain why the meniscus of water in a glass capillary tube is concave, while that of mercury is convex, relating it to cohesive and adhesive forces.
The shape of the meniscus is determined by the interplay between cohesive and adhesive forces:
- Water (Concave): The adhesive force (attraction between water and glass molecules) is stronger than the cohesive force (attraction between water molecules). This causes the water to wet and climb the glass walls, forming a concave surface.
- Mercury (Convex): The cohesive force (strong attraction between mercury atoms) is far greater than the adhesive force with glass. This causes the mercury to pull inward on itself, minimising contact with the glass and forming a convex surface.
5. What would happen if the length of the capillary tube is shorter than the height 'h' to which water would normally rise? Would the water overflow? Justify your answer.
No, the water will not overflow. This is a frequently asked HOTS question. The water will rise to the top of the available tube length, and the meniscus will adjust itself. The angle of contact will increase, and the radius of curvature of the meniscus will become larger. This change reduces the upward pull of the surface tension force until it exactly balances the weight of the shorter water column, preventing any overflow.
6. How does adding a detergent to water affect its surface tension, and why is this property important for cleaning?
Adding a detergent or soap to water significantly decreases its surface tension. This is crucial for cleaning because water with low surface tension can spread out more easily and penetrate the fine pores and fibres of a fabric. This allows the water to effectively wet dirt and grease, making it easier to wash them away.
7. If the capillary tube in the experiment is tilted at an angle, how will this affect the vertical height 'h' to which the water rises?
The vertical height 'h' to which the water rises will remain the same. While the length of the water column measured along the tilted tube will increase, the vertical height is determined by the pressure difference across the meniscus, which depends on surface tension and is balanced by the hydrostatic pressure (ρgh). This hydrostatic pressure corresponds to the vertical height 'h', which is independent of the tube's tilt.
8. Why do molecules at the surface of a liquid possess higher potential energy compared to molecules inside the liquid?
A molecule deep inside the liquid is attracted equally in all directions by neighbouring molecules, resulting in a zero net cohesive force. However, a molecule at the surface experiences a net inward pull from the molecules below it. To bring a molecule from the interior to the surface, work must be done against this net inward force. This work is stored as potential energy, giving the surface molecules a higher energy state.
9. How does temperature affect the surface tension of water? Explain the reason behind this change.
The surface tension of water decreases as the temperature increases. This is because a rise in temperature increases the kinetic energy of the water molecules. As the molecules move more vigorously, the average distance between them increases, which in turn weakens the intermolecular cohesive forces responsible for surface tension.
























