




An Overview of Class 10 Chemistry Properties Of Acids And Bases Experiment
Chemistry Experiment - Properties of Acids and Bases
We frequently employ substances that scientists refer to as acids in our daily lives. Citric acid is included in the orange or grapefruit juice you consume for breakfast (also known as vitamin C). Lactic acid is a component of soured milk. Acetic acid can be found in the vinegar used in salad dressing. In accordance with this, an acid-base combination is thought to make up a chemical bond. Thus, by splitting a molecule into its acidic and basic components, its properties can be understood.
Table of content
Aim
Reaction with Litmus Solution (Blue/Red)
Reaction with Zinc Metal
Reaction with Solid Sodium Carbonate
Result
Aim
To study the properties of Acids (Hydrochloric Acid) and Bases (Sodium Hydroxide)
Materials Required
Test tubes
Test tube stand
Test tube holder
Cork
Droppers
Flat bottom flask
Beaker
Litmus solution/paper (red and blue)
Glass rod
Zinc granules
Freshly prepared lime water
Solid sodium carbonate
Dil. HCl
Dil NaOH
Theory
An acid is a chemical like HCl that produces H+ ions when it dissolves in water. Acids do not affect red litmus, but turn blue litmus red. When HCl reacts with zinc metal, zinc chloride (ZnCl2) and hydrogen gas (H2) is released.
Zn(s)+2HCl(aq)→ZnCl2(aq)+H2(g)A pop sound and a blue flame are produced as hydrogen gas burns in the air.
2H2(g)+O2(g)→2H2O(l)
Lime water turns milky as a result of the creation of calcium carbonate when HCl combines with sodium carbonate (aqueous/solid). The milkiness vanishes if too much CO2 is added to the solution.
Na2CO3(s) + 2HCl(aq) → 2NaCl(aq) + H2O(l) + CO2(g)
Ca(OH)2(aq) + CO2(g) → CaCO3(s) + H2O(l)
CaCO3(s) + H2O(l) + CO2(g) → Ca(HCO3)2(aq)
A base is a substance which gives OH– ions when it is dissolved in water. Sodium hydroxide (NaOH) is a strong base. Its pH is much higher than 7.
So, it turns red litmus into blue and does not impact the blue litmus.
On reacting with Zn metal, it forms a salt (sodium zincate) and hydrogen gas (H2) is liberated.
Zn(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2(g)
Solid Na2CO3 does not react with NaOH because both are basic in nature.
Na2CO3(s) + 2HCl(aq) → No reaction
Base (NaOH) neutralises acid (HCl) to give salt (NaCl).
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O
Procedure
1. Litmus Test
Place two test tubes in a test tube stand with the labels A and B. Take 5 mL of the blue litmus solution and 5 mL of the red litmus solution, respectively, in test tubes A and B.
Add a few drops of HCl using a dropper to each test tube. Each tube is stirred using a different glass rod. Take note if the solutions' colour changes at all.
Again, mark two test tubes with the letters C and D. Put three millilitres of blue litmus in test tube C and three millilitres of red litmus in test tube D in a test tube stand.
Add a few drops of NaOH to each with a dropper. Take note if the liquids in the test tubes change colour in any way.
Observation
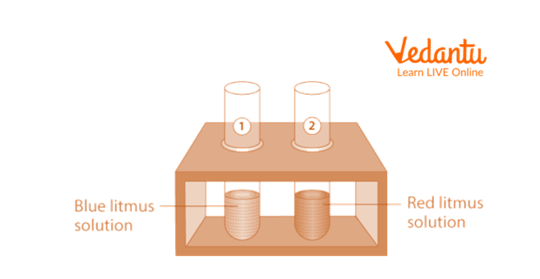
Test with Litmus
1. Reaction with Zinc (Zn) Metal
Put a few zinc granules in a test tube that is clean and dry.
Add enough HCl to the test tube holding the zinc granules to completely immerse them in the acid.
Put a cork with a glass delivery tube in place.
After two to three minutes, there will be a strong reaction and the evolution of odourless, colourless gas.
When a match is held close to the gas tube's mouth, the gas ignites with a faint blue flame that makes a popping sound.
Similarly, fill the test tube with zinc granules with NaOH until they are completely submerged.
Heat the test tube and insert a cork with a glass delivery tube.
After two to three minutes, there will be a strong reaction and the evolution of odourless, colourless gas.
When a match is held close to the gas tube's mouth, the gas ignites with a faint blue flame that makes a popping sound.
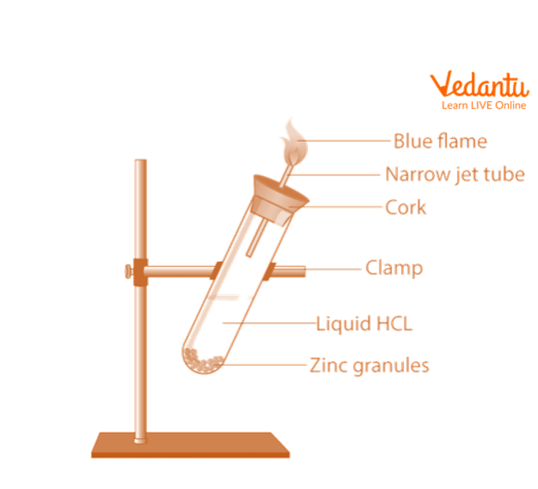
Action of NaOH with Zn metal
Reaction
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Zn(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2(g)
Observation
1. Reaction with Solid Sodium Carbonate(Na2CO3)
Take a small amount of solid sodium carbonate in a flat bottom flask and some distilled water in it, shake it well.
Take a double bore cork with a thistle funnel and a delivery tube fitted in it, fit it on the open end of the flask.
Through the thistle tube, add HCl to the flask.
A reaction has occurred, resulting in the development of odourless and colourless gas.
Then, using a delivery tube, gas is fed through freshly prepared lime water.
The lime water turns milky.
Similarly, take a small amount of Na2CO3 in a test tube.
Add some drops of dil. NaOH solution in the test tube containing Na2CO3.
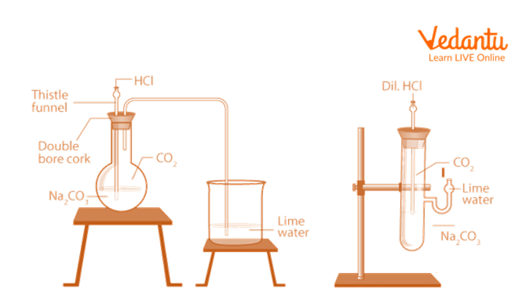
Reaction with Solid Sodium Carbonate
Reaction
Na2CO3(s)+2HCl(aq)→2NaCl(aq)+H2O(l)+CO2(g)
Ca(OH)2(aq)+CO2(g)→CaCO3(s)+H2O(l)
CaCO3(s)+H2O(l)+CO2(g)→Ca(HCO3)2(aq)
Observation
Result
From the above experiment, we can conclude that:-
Hydrochloric acid turns blue litmus solution/paper to red, but it does not affect red litmus solution/paper.
Sodium hydroxide turns red litmus solution/paper blue, but it does not affect blue litmus solution/paper.
HCl reacts with zinc metal to liberate hydrogen gas and also forms zinc chloride as a product.
NaOH reacts with zinc metal and liberates hydrogen gas. During this reaction, sodium zincate is also formed.
HCl reacts with sodium carbonate to liberate carbon dioxide. Hence, we conclude that hydrochloric acid is acidic in nature.
It does not react with sodium carbonate. Hence, we conclude that sodium hydroxide (NaOH) is basic in nature.
Precautions
As HCl is corrosive in nature, it should be handled with care.
Use small quantities of chemicals.
Use small quantities of Zn and HCl, otherwise large amounts of H2 will be formed, which may cause an explosion.
Use clean zinc metal, otherwise the reaction will occur very slowly.
Add HCl to Na2CO3, when the apparatus is airtight.
Observe the milkiness in the lime water soon.
In case you allow carbon dioxide to pass for a long time through lime water, the milkiness may be removed due to the formation of soluble calcium bicarbonate as depicted in the reaction as follows:
CaCO3(s) + CO2(g) + H2O(l) → Ca(HCO3)2(aq).
Shake the solutions and reaction mixtures carefully without spilling.
Care must be taken while performing the combustion test with H2.
Since sodium hydroxide is extremely corrosive, it should be handled carefully. Don't bring the zinc and diluted NaOH combination to a boil.
To achieve the best results, only a small amount of chemicals should be used to conduct the experiment. Before using, test tubes and droppers should be well cleaned with distilled water.
When testing with acids, alkalis, or indicators, never swap droppers.
After the experiment is finished, hands need to be carefully washed.
Lab Manual Questions
1. When exposed to a drop of dil. NaOH, what colour would blue litmus paper take on?
Ans. When the blue litmus paper is in contact with a drop of dil NaOH, its colour does not change.
2. What metals, besides Zinc, react with alkalis to create hydrogen gas? What is the name of these metals?
Ans. Aluminium is a metal, besides Zinc, that reacts with alkalis to create hydrogen gas. These metals are called Amphoteric oxides
3. A blue piece of litmus paper was dipped in dilute HCl solution. Which colour do you think you would see on the litmus paper?
Ans. Dilute HCl turns blue litmus paper red.
4. What impact will litmus have on hydrogen gas?
Ans. Hydrogen gas is neutral towards litmus. It is neither acidic nor basic therefore, it neither changes the colour of blue litmus nor red litmus.
Viva Questions
1. When carbon dioxide is passed through lime water, it turns milky. Due to which insoluble substance milkiness is formed?
Ans. Insoluble calcium carbonate (CaCO3) makes the solution milky.
2. What would happen if dilute HCl and dilute NaOH were combined in an equal amount? Also, write the equation.
Ans. Equal volumes of dilute HCl and dilute NaOH cause a neutralisation reaction that results in the production of salt and water. The reaction between an acid and a base is given below:
HCl(aq) + NaOH(aq) → NaCl(aq) + H2O(l)
3. How do acids and bases react with metals?
Ans. Acids react with most metals to form a salt and hydrogen gas. For example, zinc metal reacts with hydrochloric acid, producing zinc chloride and hydrogen gas.
Zn(s) + 2HCl(aq) → ZnCl2(aq) + H2(g)
Bases also react with certain metals, like zinc or aluminium, to produce hydrogen gas. For example, sodium hydroxide reacts with zinc and water to create sodium zincate and hydrogen gas.
Zn(s) + 2NaOH(aq) → Na2ZnO2(aq) + H2(g)
4. What substance is formed from the reaction of aluminium metal with NaOH solution?
Ans. Aluminium metal reacts with NaOH solution to produce sodium meta-aluminate:
2NaOH(aq) + 2H2O(l) + 2Al(s) → 2NaAlO2(aq) + 3H2(g)
5. What are strong bases?
Ans. Bases which dissociate into their ions completely are called strong bases. Example: NaOH, KOH etc.
6. What are strong acids?
Ans. Acids which dissociate into their ions completely are called strong acids. Example: HCl, H2SO4 etc.
7. What do you understand about weak acids?
Ans. A weak acid is an acid that partially dissociates into its ions in an aqueous solution or water. Example: Acetic acid, Citric Acid etc.
8. What are the characteristics of bases
Ans. When dissolved in water, bases:
Conduct electricity
Change red litmus to blue
Have a slippery feeling
React with acids to neutralize their properties
9. What are the characteristics of Acids?
Ans. When dissolved in water, acids:
Conduct electricity
Change blue litmus to red
Have a sour taste
React with bases to neutralize their properties
10. What do you understand by weak base?
Ans. Weak Bases are the bases that partially dissociate in water. Example Ammonia, Aluminium Hydroxide etc.
Practical Questions
What acids and bases have in common?
Conduct electricity in aqueous solution
Have same taste
Have same ions
Have same indication results on reacting with Litmus
Ans. Both acids and bases conduct electricity in aqueous solution
Which of the following is not a property shown by acids?
Have Sour taste
Turns blue litmus red
Turns red litmus blue
Gives H+ in water
Ans. All acids turn blue litmus red.
Which describes the chemical property of acids?
Sour taste
Acids react with carbonates and hydrogen carbonates to form a salt, water, and carbon dioxide gas.
Feel slippery
None of the above
Ans. Acids react with carbonates and hydrogen carbonates to form a salt, water, and carbon dioxide gas.
Which statement is true about weak acids
Completely dissociated in water
Partially dissociation in water
No reaction observed
Very corrosive
Ans. Weak acids are acids that are partially dissociated in water
Which statement is true about weak bases
Completely dissociated in water
Partially dissociation in water
No reaction observed
Very corrosive
Ans. Weak bases are bases that are partially dissociated in water
Which is an example of Strong Acids
H2SO4
CH3COOH
C6H5COOH
H3PO4
Ans. H2SO4 is an example of strong acids
Which is an example of Weak Bases
NaOH
KOH
LiOH
NH3
Ans. NH3 is an example of weak base
Which is an example of Weak Acids
C6H5COOH
H2SO4
HCl
HNO3
Ans. C6H5COOH is an example of weak acids.
Which is an example of Strong Bases
NH3
Al(OH)3
Zn(OH)2
KOH
Ans. KOH is an example of Strong Bases
Why Acetic Acid is considered as a weak acid
Highly ionised
Contains COOH group
Partially ionised
Aqueous solution is acidic
Ans. Acetic Acid is considered as a weak acid because it is partially ionised
Conclusion
Typically, sour tastes help to identify acidic chemicals. A fundamental definition of an acid is a molecule that has the ability to donate an H+ ion and maintain its energetic favorability even after losing H+. Acids are known to turn blue litmus paper red. On the other hand, bases have a bitter flavour and are slippery to touch. Bases have the ability to donate OH- and can turn red litmus blue.
FAQs on Class 10 Chemistry Properties Of Acids And Bases Experiment
1. What are the key differences between acids and bases that are important for the CBSE Class 10 exam 2025-26?
For the board exam, it's crucial to know the fundamental differences between acids and bases. The most important distinctions are:
- Taste: Acids have a sour taste, while bases have a bitter taste.
- Litmus Test: Acids turn blue litmus paper red, whereas bases turn red litmus paper blue. This is a key identification test.
- Ionisation in Water: In an aqueous solution, acids dissociate to produce hydrogen ions (H⁺). Bases dissociate to produce hydroxide ions (OH⁻).
- Feel: Bases feel soapy or slippery to the touch, while acids do not have this property.
2. Explain with a balanced chemical equation what happens when an acid reacts with a metal carbonate. Why is this considered an important reaction?
This is a frequently asked question in board exams. When an acid reacts with a metal carbonate, it forms a corresponding salt, water, and carbon dioxide gas. The gas evolved (CO₂) produces a characteristic brisk effervescence. The general equation is:
Acid + Metal Carbonate → Salt + Water + Carbon Dioxide
For example, when dilute hydrochloric acid reacts with sodium carbonate:
2HCl(aq) + Na₂CO₃(s) → 2NaCl(aq) + H₂O(l) + CO₂(g)
This reaction is important as it serves as a test for both acids and carbonates.
3. What is a neutralisation reaction? Provide two examples of its application in daily life that are important for exam questions.
A neutralisation reaction is a chemical reaction in which an acid and a base react quantitatively with each other to form a salt and water. It is an essential concept for 3-mark questions.
Example Equation: HCl (Acid) + NaOH (Base) → NaCl (Salt) + H₂O (Water)
Two important applications for the CBSE exam are:
- Treating Indigestion: Our stomach produces hydrochloric acid. Excess acid causes indigestion. We take antacids like Milk of Magnesia (magnesium hydroxide), which is a mild base, to neutralise the excess acid and get relief.
- Soil Treatment: Farmers often add bases like slaked lime (calcium hydroxide) or chalk (calcium carbonate) to acidic soil. This neutralises the soil's acidity, making it suitable for plant growth.
4. What is the key distinction between a strong acid and a weak acid? Give one example for each.
The primary distinction lies in their degree of ionisation in water. A strong acid is an acid that ionises completely or almost completely in an aqueous solution, producing a high concentration of H⁺ ions. A weak acid, on the other hand, ionises only partially in an aqueous solution, resulting in a lower concentration of H⁺ ions.
- Example of a Strong Acid: Hydrochloric acid (HCl)
- Example of a Weak Acid: Acetic acid (CH₃COOH)
5. Why do substances like HCl and H₂SO₄ exhibit acidic properties only in an aqueous solution, while solutions of glucose or alcohol do not, despite also containing hydrogen?
This is a high-order thinking (HOTS) question. The acidic character of a substance is due to the presence of free hydrogen ions (H⁺).
In an aqueous solution, compounds like HCl and H₂SO₄ dissociate to release H⁺ ions. However, in compounds like glucose (C₆H₁₂O₆) and alcohol (C₂H₅OH), the hydrogen atoms are bonded covalently and do not ionise or separate as H⁺ ions when dissolved in water. Therefore, despite the presence of hydrogen in their molecules, they do not show acidic properties.
6. You are given three unlabelled test tubes containing distilled water, an acidic solution, and a basic solution. If you are only provided with a strip of red litmus paper, how would you identify the contents of each test tube?
This is a classic practical-skill-based question. The identification can be done in three steps:
- Dip the red litmus paper into each of the three test tubes. The solution that turns the red litmus blue is the basic solution.
- Now, use this newly blue litmus paper to test the remaining two solutions. The solution that turns this blue litmus paper back to red is the acidic solution.
- The solution that causes no change in either the initial red litmus paper or the blue litmus paper is distilled water, as it is neutral.
7. Why does tooth decay start when the pH of the mouth falls below 5.5? How does using toothpaste help in preventing it?
This is an important application-based question from the NCERT syllabus. Tooth enamel is the hardest substance in the body and is made of calcium phosphate. It does not dissolve in water but gets corroded when the pH in the mouth falls below 5.5. Bacteria present in the mouth produce acids by degrading sugar and food particles. When the pH drops, this acid attacks and corrodes the enamel, leading to tooth decay.
Toothpastes are generally basic (alkaline) in nature. They help by neutralising the excess acid produced in the mouth, thus preventing tooth decay.
8. A metal compound 'A' reacts with dilute hydrochloric acid to produce brisk effervescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation if one of the products is calcium chloride. What type of compound is 'A'?
This is a typical competency-based question for the board exam.
Analysis:
- The gas that produces 'brisk effervescence' and 'extinguishes a burning candle' is Carbon Dioxide (CO₂).
- Carbon dioxide is produced when an acid reacts with a metal carbonate or a metal hydrogencarbonate.
- Since the salt formed is calcium chloride (CaCl₂), the metal in the compound 'A' must be calcium (Ca).
The metal compound 'A' is Calcium Carbonate (CaCO₃).
Balanced Chemical Equation:
CaCO₃(s) + 2HCl(aq) → CaCl₂(aq) + H₂O(l) + CO₂(g)






































