
Structure of ammonia is:
A) Trigonal
B) Tetrahedral
C) Pyramidal
D)Trigonal pyramidal
Answer
233.1k+ views
Hint: The VSEPR theory helps in the prediction of the shape of molecules. This theory predicts shape on the basis of lone pairs and bond pairs surrounding the central atom. Here, we will use the VSEPR theory to identify ammonia's shape.
Complete Step by Step Answer:
Let's discuss the structure of ammonia. The ammonia is written by the formula of \[{\rm{N}}{{\rm{H}}_{\rm{3}}}\]. The nitrogen atom forms three bond pairs with the hydrogen atom. The count of valence electrons of the N atom in ammonia is five, and three electrons are used in the formation of covalent bonds with H atoms. And one lone pair is present.
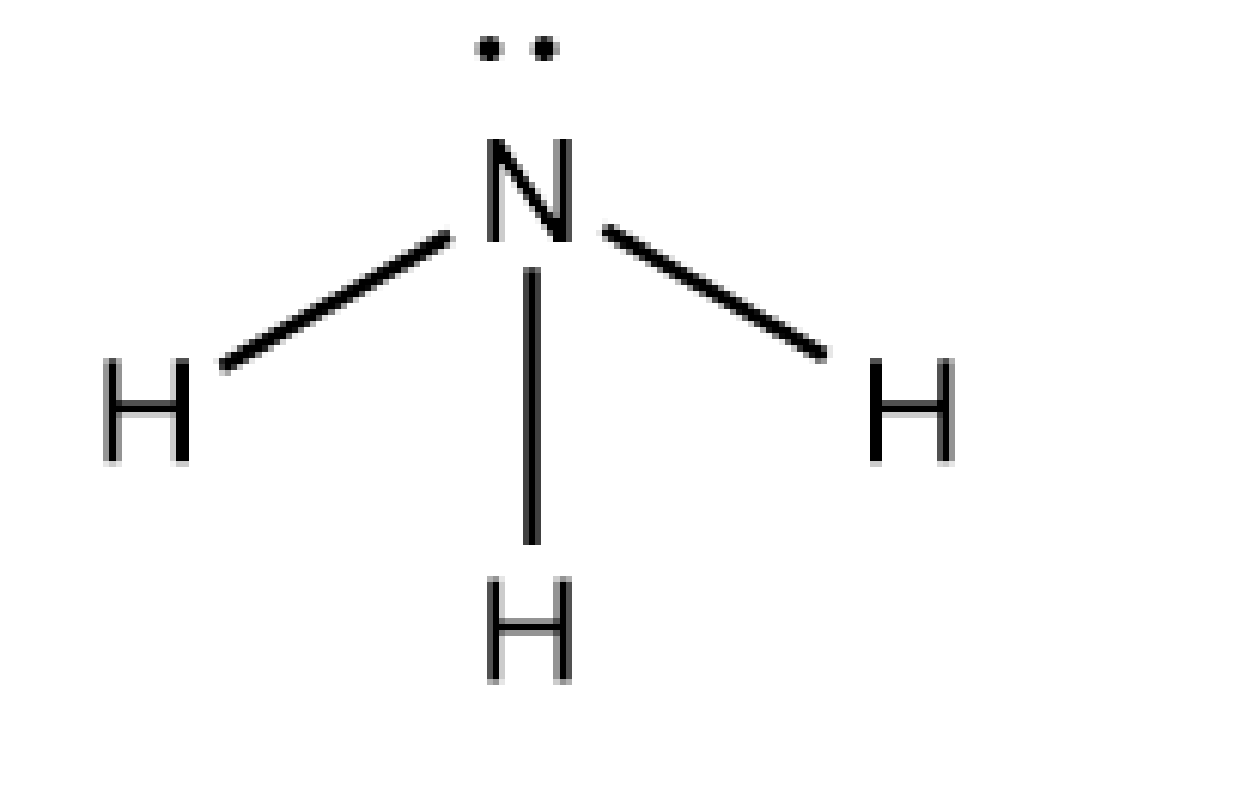
Image: Structure of ammonia
Let's discuss the electron pair geometry of ammonia. As the molecule has three numbers of bond pairs and one lone pair, the electron pair geometry of ammonia is tetrahedral. As one lone pair and three numbers of bond pairs are present, the molecular shape of the ammonia molecule is trigonal bipyramidal.
Hence, option D is right.
Additional Information: Ammonia is a gas that has no colour. It possesses a distinct smell. It is a nitrogenous waste among aquatic organisms. It is an important component of many fertilisers. The pure form of ammonia can be directly applied to soil for fertility.
Note: If the electron pair geometry is tetrahedral, there are three possibilities, if no lone pair is present, then the shape of the molecule is tetrahedral. If one lone pair is present, the shape is trigonal bipyramidal (ammonia) and if two lone pairs are present, the shape is bent.
Complete Step by Step Answer:
Let's discuss the structure of ammonia. The ammonia is written by the formula of \[{\rm{N}}{{\rm{H}}_{\rm{3}}}\]. The nitrogen atom forms three bond pairs with the hydrogen atom. The count of valence electrons of the N atom in ammonia is five, and three electrons are used in the formation of covalent bonds with H atoms. And one lone pair is present.

Image: Structure of ammonia
Let's discuss the electron pair geometry of ammonia. As the molecule has three numbers of bond pairs and one lone pair, the electron pair geometry of ammonia is tetrahedral. As one lone pair and three numbers of bond pairs are present, the molecular shape of the ammonia molecule is trigonal bipyramidal.
Hence, option D is right.
Additional Information: Ammonia is a gas that has no colour. It possesses a distinct smell. It is a nitrogenous waste among aquatic organisms. It is an important component of many fertilisers. The pure form of ammonia can be directly applied to soil for fertility.
Note: If the electron pair geometry is tetrahedral, there are three possibilities, if no lone pair is present, then the shape of the molecule is tetrahedral. If one lone pair is present, the shape is trigonal bipyramidal (ammonia) and if two lone pairs are present, the shape is bent.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




