
In the anomalous expansion of water, at what temperature, the density of water is maximum and what is the value of density at that temperature?
A. 4°C and 1.0 gm/$cm^3$
B. <4°C and 0.4 gm/$cm^3$
C. >4°C and 0.4 gm/$cm^3$
D. 10°C and 0.99 gm/$cm^3$
Answer
537.6k+ views
Hint: Expansion coefficient becomes negative when the temperature of the water is in the range of 0°C
Complete answer:
It is the general rule for most of the substances that the volume will increase as you heat it. This general rule holds true above 4°C. When you are cooling the temperature from a high temperature to 4°C, the movement of the water molecules gets restricted and they start to come close to each other.
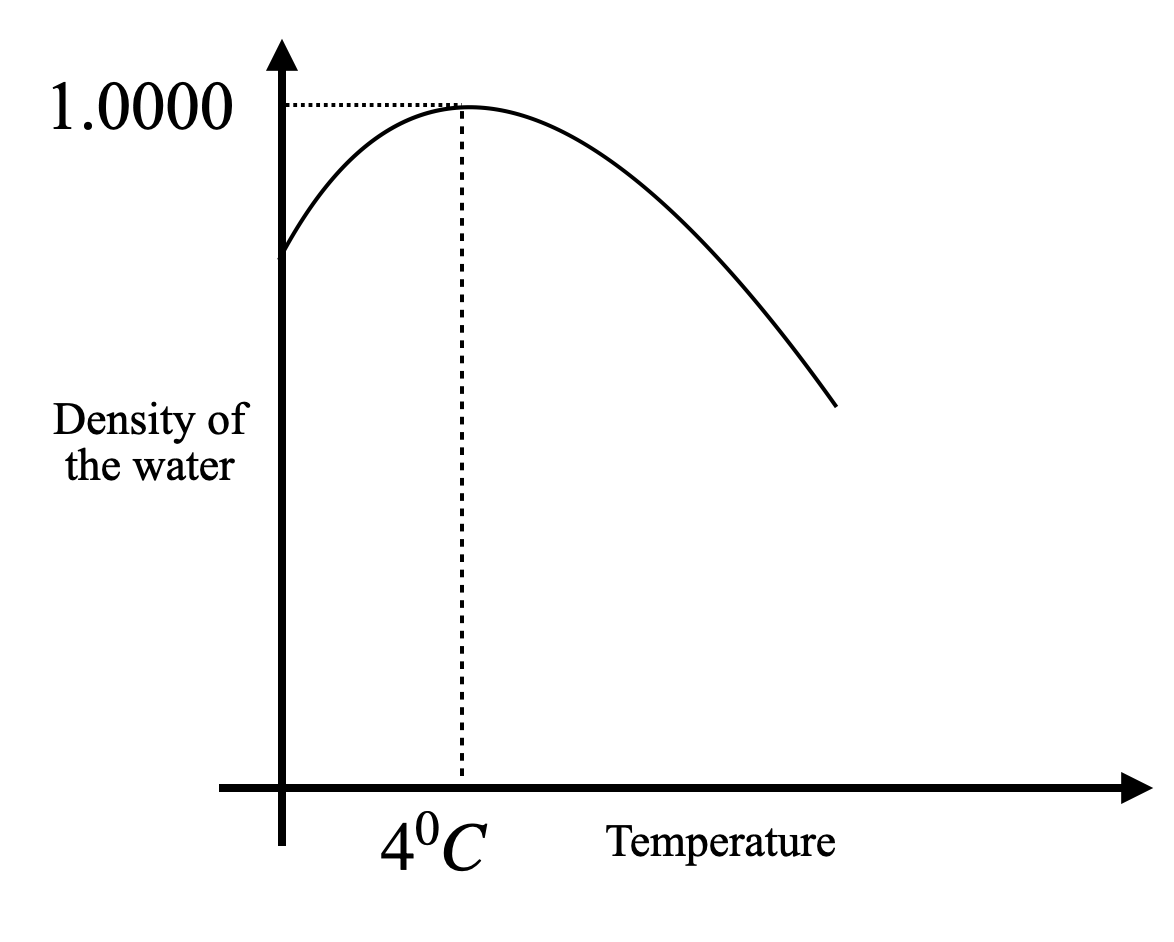
As a result, the volume of the water decreases and the density of water increases at the same time. So, as we can see in the plot, the density of the water reaches its maximum at 4°C. However, the density starts to decrease as we cool it further.
Let us look at the reason behind this phenomenon. When the temperature of the water is more than 4°C, the molecules are attracted because of the O-O polar attraction. However, as the volume of the water has reached the minimum value at 4°C, H-O interactions take over. This interaction is not powerful enough to contact the molecules any further. That is why it experiences an expansion, rather than a contraction.
As a result, the volume expansion coefficient of water is negative in the range of
0°CThe volume increases as we reduce the temperature from 4℃. As a result, we have a maximum density at 4°C and density will be 1.0 gm/$cm^3$
So, the correct choice is option (A).
Note:
This phenomenon helps the water bodies to bear life even when the surface of the lake/river is completely frozen. As the density of water is maximum at 4°C, it goes at the bottom of the water body. Water with lower temperature stays at the top and is frozen. However, the bottom stays at a comfortable 4°C.
Complete answer:
It is the general rule for most of the substances that the volume will increase as you heat it. This general rule holds true above 4°C. When you are cooling the temperature from a high temperature to 4°C, the movement of the water molecules gets restricted and they start to come close to each other.

As a result, the volume of the water decreases and the density of water increases at the same time. So, as we can see in the plot, the density of the water reaches its maximum at 4°C. However, the density starts to decrease as we cool it further.
Let us look at the reason behind this phenomenon. When the temperature of the water is more than 4°C, the molecules are attracted because of the O-O polar attraction. However, as the volume of the water has reached the minimum value at 4°C, H-O interactions take over. This interaction is not powerful enough to contact the molecules any further. That is why it experiences an expansion, rather than a contraction.
As a result, the volume expansion coefficient of water is negative in the range of
0°C
So, the correct choice is option (A).
Note:
This phenomenon helps the water bodies to bear life even when the surface of the lake/river is completely frozen. As the density of water is maximum at 4°C, it goes at the bottom of the water body. Water with lower temperature stays at the top and is frozen. However, the bottom stays at a comfortable 4°C.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




