
In Raman scattering, stokes and anti-stokes lines respectively represent lines with _____ and _____ wavelength.
Answer
585.6k+ views
Hint:In Raman scattering the energy increase or decrease from the excitation is related to the vibrational energy spacing in the ground electronic state of the molecule and as a result of these energy differences we can obtain
Complete Step by Step Answer:
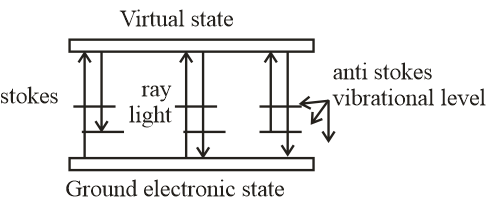
It is the shift in wavelength of the elastically scattered radiation that produces the chemical and structural information remaining shifted photons can be of either higher or lower energy, depending upon the vibration state of the molecule under study. According to energy diagram stokes radiation occurs at lower energy than the ray light radiation and anti-stokes radiation has greater energy.The energy increase or decrease is related to the vibrational energy levels in the ground electronic state of molecules.
The energy of the scattered radiation is less than the incident radiation for the stokes line and the energy of the scattered radiation is more than the incident radiation for the anti-stokes line. Therefore, the wavelength of the strokes and anti-stokes lines are a direct measure of the vibrational energies of the molecule.Hence, the stroke line has high wavelengths and anti-stokes lines has least wavelength.
Note: In Raman scattering, the more intense strokes lines are normally measured. Raman scattering is a relatively weak process. The number of photons in raman scattering is quite small.
Complete Step by Step Answer:

It is the shift in wavelength of the elastically scattered radiation that produces the chemical and structural information remaining shifted photons can be of either higher or lower energy, depending upon the vibration state of the molecule under study. According to energy diagram stokes radiation occurs at lower energy than the ray light radiation and anti-stokes radiation has greater energy.The energy increase or decrease is related to the vibrational energy levels in the ground electronic state of molecules.
The energy of the scattered radiation is less than the incident radiation for the stokes line and the energy of the scattered radiation is more than the incident radiation for the anti-stokes line. Therefore, the wavelength of the strokes and anti-stokes lines are a direct measure of the vibrational energies of the molecule.Hence, the stroke line has high wavelengths and anti-stokes lines has least wavelength.
Note: In Raman scattering, the more intense strokes lines are normally measured. Raman scattering is a relatively weak process. The number of photons in raman scattering is quite small.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




