
Explain Raman effect with the help of energy level diagram.
Answer
598.5k+ views
Hint: The scattered monochromatic light contains some additional frequencies other than that of incident frequency. This phenomenon is known as the Raman effect. This occurs either due to the absorption of energy by incident photons or due to disbanding of energy by it.
Complete step by step answer:
When monochromatic light is allowed to pass through a substance it gets scattered. After undergoing scattering, this light then contains some additional frequencies other than the ones it had as an incident frequency. This phenomenon of the addition of additional frequencies to a scattered monochromatic light is called Raman Effect.
The lines whose frequencies get modified in the Raman effect are known as Raman lines. The lines which have frequencies lower than the incident frequency itself are called Stoke's lines. Additionally, and the lines which have frequencies higher than the incident frequency itself are referred to as Anti-stokes lines.
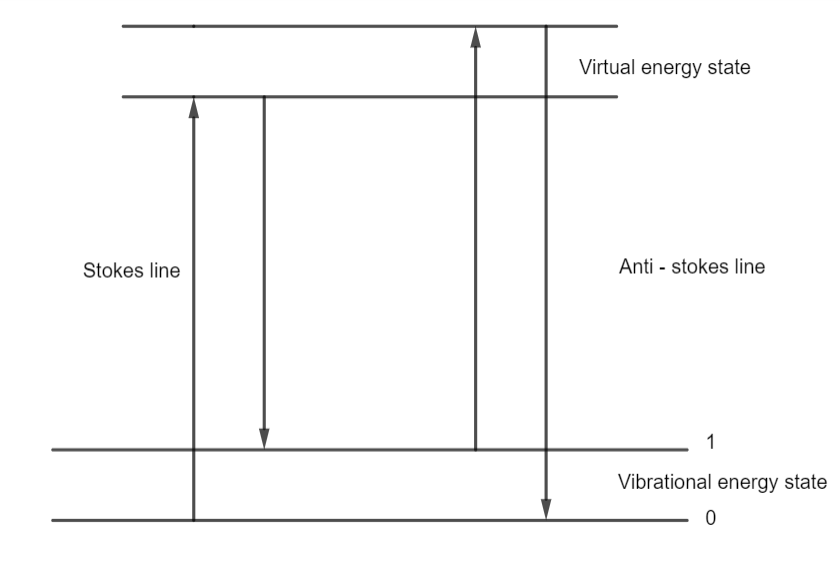
To understand the Raman effect consider the scattering of photons of incident light by atoms or molecules. Let the photons of incident light have their energy $h{v_ \circ }$.
If an atom or a molecule of a liquid is struck by the photon, then a part of its energy may get used to excite that atom of the liquid and the rest gets scattered. Therefore, as a consequence, the spectral line will have lower frequency than the original incident frequency. This will be called Stoke's line.
If an atom or a molecule of a liquid is struck by the photon which is in an excited state, the scattered photon will gain energy in this case. Therefore, as a consequence the spectral line will have higher frequency than the original incident frequency. This will be called the Anti-Stoke 's line.
It is also a possibility that when a light photon strikes an atom or a molecule, the scattering of the photon may be elastic in nature. So neither there is a gain of energy or loss of energy. Therefore, the result would be an unmodified frequency.
Raman shifts are reported in wavenumber $\left( {c{m^{ - 1}}} \right)$. Its equation if ${\lambda _ \circ }$ is the laser wavelength, ${\lambda _R}$ is the Raman radiation wavelength and $\Delta $ represent Raman shift is given as:
$\Delta \left( {c{m^{ - 1}}} \right) = \left( {\dfrac{1}{{{\lambda _ \circ }}} - \dfrac{1}{{{\lambda _R}}}} \right)$
Note: Raman Effect has very important applications. This is because it can be used in any field that requires microscopic, non-destructive, chemical analysis, and imaging. It can provide data in both qualitative or quantitative form.
Complete step by step answer:
When monochromatic light is allowed to pass through a substance it gets scattered. After undergoing scattering, this light then contains some additional frequencies other than the ones it had as an incident frequency. This phenomenon of the addition of additional frequencies to a scattered monochromatic light is called Raman Effect.
The lines whose frequencies get modified in the Raman effect are known as Raman lines. The lines which have frequencies lower than the incident frequency itself are called Stoke's lines. Additionally, and the lines which have frequencies higher than the incident frequency itself are referred to as Anti-stokes lines.

To understand the Raman effect consider the scattering of photons of incident light by atoms or molecules. Let the photons of incident light have their energy $h{v_ \circ }$.
If an atom or a molecule of a liquid is struck by the photon, then a part of its energy may get used to excite that atom of the liquid and the rest gets scattered. Therefore, as a consequence, the spectral line will have lower frequency than the original incident frequency. This will be called Stoke's line.
If an atom or a molecule of a liquid is struck by the photon which is in an excited state, the scattered photon will gain energy in this case. Therefore, as a consequence the spectral line will have higher frequency than the original incident frequency. This will be called the Anti-Stoke 's line.
It is also a possibility that when a light photon strikes an atom or a molecule, the scattering of the photon may be elastic in nature. So neither there is a gain of energy or loss of energy. Therefore, the result would be an unmodified frequency.
Raman shifts are reported in wavenumber $\left( {c{m^{ - 1}}} \right)$. Its equation if ${\lambda _ \circ }$ is the laser wavelength, ${\lambda _R}$ is the Raman radiation wavelength and $\Delta $ represent Raman shift is given as:
$\Delta \left( {c{m^{ - 1}}} \right) = \left( {\dfrac{1}{{{\lambda _ \circ }}} - \dfrac{1}{{{\lambda _R}}}} \right)$
Note: Raman Effect has very important applications. This is because it can be used in any field that requires microscopic, non-destructive, chemical analysis, and imaging. It can provide data in both qualitative or quantitative form.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




