
Compound (A), ${{C}_{8}}{{H}_{9}}Br$, gives a pale yellow color precipitate when warmed with alcoholic $AgN{{O}_{3}}$. Oxidation of (A) gives an acid (B), ${{C}_{8}}{{H}_{6}}{{O}_{4}}$. (B) easily forms anhydride on heating. Identify the compound (A).
a.
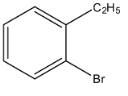
b.
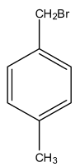
c.
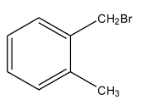
d.
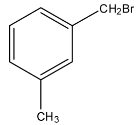




Answer
535.8k+ views
Hint: In the question, the compound (A) can form a yellow precipitate with silver nitrate, this is due to the fact that there is the formation of silver bromide, this can be formed only when the bromine atom from the compound can be easily released, if the bromine atom is attached with the benzene ring then, it will not easily release.
Complete answer: In the question, the compound (A) can form a yellow precipitate with silver nitrate, this is due to the fact that there is the formation of silver bromide, this can be formed only when the bromine atom from the compound can be easily released, if the bromine atom is attached with the benzene ring then, it will not easily release.
So, from the given options, (a) is not correct.
The second part of the question says that oxidation forms acid and it can easily form the anhydride. An anhydride can be formed from the compound only if both the acid groups are very near. Therefore, from the given options, only option (c), can form the anhydride.
The reactions are given below:
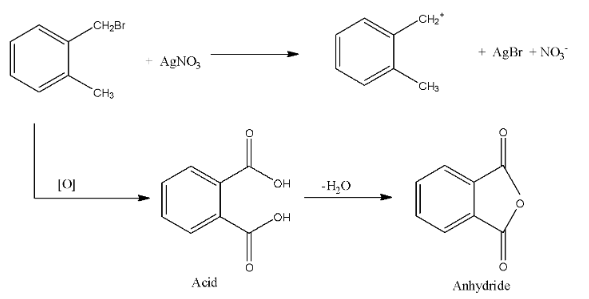
So, from the reaction, we can see that a correct answer is an option (c).
Note: If the acids on the benzene ring are present on the meta and para positions then, the anhydride cannot be formed, and anhydride is formed when one molecule of water is removed from the acid.
Complete answer: In the question, the compound (A) can form a yellow precipitate with silver nitrate, this is due to the fact that there is the formation of silver bromide, this can be formed only when the bromine atom from the compound can be easily released, if the bromine atom is attached with the benzene ring then, it will not easily release.
So, from the given options, (a) is not correct.
The second part of the question says that oxidation forms acid and it can easily form the anhydride. An anhydride can be formed from the compound only if both the acid groups are very near. Therefore, from the given options, only option (c), can form the anhydride.
The reactions are given below:

So, from the reaction, we can see that a correct answer is an option (c).
Note: If the acids on the benzene ring are present on the meta and para positions then, the anhydride cannot be formed, and anhydride is formed when one molecule of water is removed from the acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




