
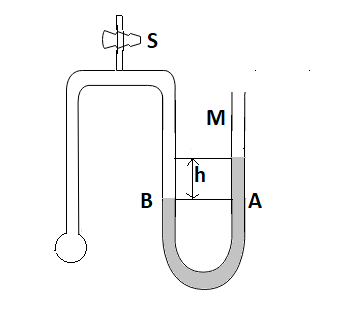
A tube of fine bore AB is connected to a manometer M as shown. The stopcock S controls the flow of air AB is dipped into a liquid whose surface tension is σ. On opening the stopcock for a while a bubble is formed at B and the manometer level is recorded showing a difference h in the levels in the two arms if P be the density of manometer liquid and r the radius of curvature of the bubble then the surface tension σ of the liquid is given by
(A). $\rho hrg$
(B). $2\rho hrg$
(C). $4\rho hrg$
(D). $\dfrac{rh\rho g}{4}$

Answer
599.1k+ views
Hint: Use concept of surface tension to make this question clearer. Consider two cases before and after the stopcock opens up. Consider atmospheric pressure too. The atmosphere will always be there because the end of the manometer is open.
Complete step by step answer:
To solve this type of question, first understand what question you want to convey.
Explanation of question: we have a narrow tube which is connected by a manometer. S is the stopcock which controls flow of air. As is dipped in the water whose surface tension is $\sigma $. When the stopcock is opened up and a bubble is formed at B. As soon as the stopcock is opened up M changes its level and changes with height h in both arms of M.
Diagram:
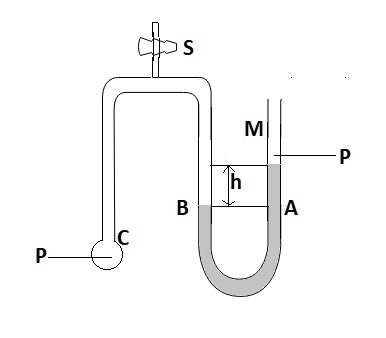
Let’s A and B be the point on M. Let’s P be the atmospheric pressure and C be the point at B where the bubble is formed.
Let’s consider the stopcock is not open so pressure at A and B is the same because so the pressure drop won’t be there in the pile.
So ${{P}_{A}}={{P}_{B}}$ or ${{P}_{A}}={{P}_{B}}={{P}_{C}}$ -------------(1)
Now open stopcock and then bubble will form at C. So pressure at A is
${{P}_{A}}=P=\rho gh$ ------------(2)
We know that it is a soap bubble. Therefore it comes under two films. Pressure at c will be greater. Therefore pressure at C is
$\begin{align}
& {{P}_{C}}-P=\dfrac{4\sigma }{r} \\
& {{P}_{C}}=P+\dfrac{4\sigma }{r} \\
\end{align}$-----------(3)
So from 1, 2 and 3, we can write that,
$\begin{align}
& P+\dfrac{4\sigma }{r}=P+hg\rho \\
& \sigma =\dfrac{rh\rho g}{4} \\
\end{align}$
Hence, option (D) is correct.
Note: Just apply a formula of pressure. Do not get confused between pressure differences. Here is a tip we should know that whenever a process is done in any experiment or in a problem then in that case take two cases. First case must be before the process and the other must be after the process and then solve.
Complete step by step answer:
To solve this type of question, first understand what question you want to convey.
Explanation of question: we have a narrow tube which is connected by a manometer. S is the stopcock which controls flow of air. As is dipped in the water whose surface tension is $\sigma $. When the stopcock is opened up and a bubble is formed at B. As soon as the stopcock is opened up M changes its level and changes with height h in both arms of M.
Diagram:

Let’s A and B be the point on M. Let’s P be the atmospheric pressure and C be the point at B where the bubble is formed.
Let’s consider the stopcock is not open so pressure at A and B is the same because so the pressure drop won’t be there in the pile.
So ${{P}_{A}}={{P}_{B}}$ or ${{P}_{A}}={{P}_{B}}={{P}_{C}}$ -------------(1)
Now open stopcock and then bubble will form at C. So pressure at A is
${{P}_{A}}=P=\rho gh$ ------------(2)
We know that it is a soap bubble. Therefore it comes under two films. Pressure at c will be greater. Therefore pressure at C is
$\begin{align}
& {{P}_{C}}-P=\dfrac{4\sigma }{r} \\
& {{P}_{C}}=P+\dfrac{4\sigma }{r} \\
\end{align}$-----------(3)
So from 1, 2 and 3, we can write that,
$\begin{align}
& P+\dfrac{4\sigma }{r}=P+hg\rho \\
& \sigma =\dfrac{rh\rho g}{4} \\
\end{align}$
Hence, option (D) is correct.
Note: Just apply a formula of pressure. Do not get confused between pressure differences. Here is a tip we should know that whenever a process is done in any experiment or in a problem then in that case take two cases. First case must be before the process and the other must be after the process and then solve.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




