
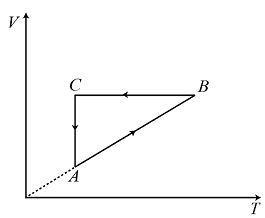
A cyclic process ABCA is shown in the volume – temperature diagram. Process on the
volume – temperature diagram is:
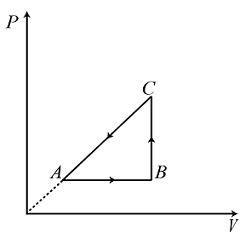
A.
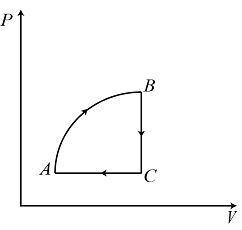
B.
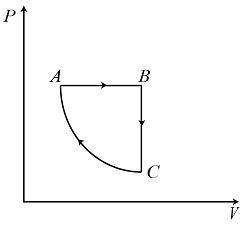
C.
D.





Answer
585.3k+ views
Hint:The process AB represents isobaric process, BC process represents isochoric process and CA process represents isothermal process.
Now, see the graph which matches these conditions.
Complete step-by-step solution:
The thermodynamic process which occurs when the pressure remains constant is called the Isobaric process. When the volume is expanded or contracted which neutralizes the pressure caused by the transfer of heat, then the isobaric process is obtained. In this process, there are some internal energy changes. In the volume – temperature graph, during this process volume will increase with increase in temperature.
In a thermodynamic process, when the volume remains constant then this process is said to be an isochoric process. The volume in this process is constant, therefore, work done by the system is zero. As in this process volume is constant, so in volume – temperature graph volume will be unchanged and temperature varies.
The process in which the temperature of the thermodynamic system remains constant then, this thermodynamic process is called isothermal process. This process has temperature as constant, therefore, in the volume – temperature graph the temperature will be unchanged and volume will change.
According to the question, in the volume – temperature graph AB is inclined straight line, in line BC the volume does not change and temperature increases and in the line CA the temperature is constant and volume varies.
Therefore, AB represents isobaric process, BC represents isochoric process and CA represents isothermal process.
The option (C) satisfies all the above criteria therefore, the correct option is (C).
Note:- There are two possible outcomes of an isobaric process:
->The system does the positive work when the system expands.
->The system does the negative work when the system contracts.
Now, see the graph which matches these conditions.
Complete step-by-step solution:
The thermodynamic process which occurs when the pressure remains constant is called the Isobaric process. When the volume is expanded or contracted which neutralizes the pressure caused by the transfer of heat, then the isobaric process is obtained. In this process, there are some internal energy changes. In the volume – temperature graph, during this process volume will increase with increase in temperature.
In a thermodynamic process, when the volume remains constant then this process is said to be an isochoric process. The volume in this process is constant, therefore, work done by the system is zero. As in this process volume is constant, so in volume – temperature graph volume will be unchanged and temperature varies.
The process in which the temperature of the thermodynamic system remains constant then, this thermodynamic process is called isothermal process. This process has temperature as constant, therefore, in the volume – temperature graph the temperature will be unchanged and volume will change.
According to the question, in the volume – temperature graph AB is inclined straight line, in line BC the volume does not change and temperature increases and in the line CA the temperature is constant and volume varies.
Therefore, AB represents isobaric process, BC represents isochoric process and CA represents isothermal process.
The option (C) satisfies all the above criteria therefore, the correct option is (C).
Note:- There are two possible outcomes of an isobaric process:
->The system does the positive work when the system expands.
->The system does the negative work when the system contracts.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




