




Understanding Semiconductors: Types, Properties, and Uses
Semiconductors are materials with electrical conductivity that lie between conductors (like metals) and insulators (such as ceramics). Their unique properties make them essential components in nearly all modern electronics. Semiconductors can be either pure elements, like silicon and germanium, or compounds like gallium arsenide. This guide will help you understand what semiconductors are, how they function, their types, and their crucial applications in everyday technology.
What are Semiconductors?
Semiconductors are materials that can conduct electricity, but not as efficiently as metals. They have a highly sensitive conductivity to external conditions such as temperature or light, making them ideal for use in electronic devices.
Some Common Semiconductors Include:
Silicon (Si) – Widely used in microchips, solar cells, and electronics.
Germanium (Ge) – Often used in transistors and diodes.
Gallium Arsenide (GaAs) – Used in high-speed electronics like solar cells and laser diodes.
Key Concepts of Semiconductors
Holes and Electrons in Semiconductors
Electrons are negatively charged particles that move in the conduction band of a semiconductor.
Holes are the absence of electrons in the valence band, acting as positive charge carriers.
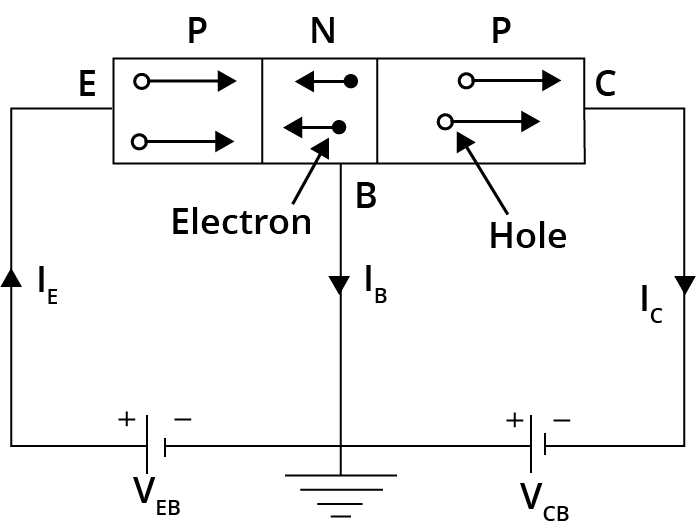
Both holes and electrons are essential in carrying current in semiconductors. However, the mobility of electrons is higher than that of holes because electrons travel more freely through the conduction band.
Mobility of Electrons and Holes
In semiconductors, electrons typically have higher mobility than holes, mainly due to differences in their band structures and scattering mechanisms.
Electrons move through the conduction band, while holes travel in the valence band. When an electric field is applied, holes face more restricted movement compared to electrons. This is because electrons when excited from their inner shells to higher energy levels, create holes in the semiconductor. The holes experience stronger attractive forces from the nucleus than electrons do, which results in lower mobility for holes.
The mobility of a particle in a semiconductor increases when:
The effective mass of the particle is smaller.
The time between scattering events is longer.
For intrinsic silicon at 300 K, the electron mobility is 1500 cm²/(V∙s), while the hole mobility is 475 cm²/(V∙s).
In a bond model of silicon (which has a valency of 4), when a free electron (represented by blue dots) leaves its lattice position, it creates a hole (represented by grey dots). This hole, which has a positive charge, can be thought of as a positive charge carrier moving through the lattice.
Band Theory of Semiconductors
Band theory explains how electrons are arranged in a solid. It divides the energy levels of electrons into bands:
Valence Band: The highest energy band filled with electrons.
Conduction Band: The empty or partially filled band where electrons can move freely to conduct electricity.
In semiconductors, the gap between the valence and conduction bands (called the band gap) is small enough for electrons to jump from the valence band to the conduction band when supplied with external energy (e.g., heat or light).
Properties of Semiconductors
Conductivity: Semiconductors conduct electricity under certain conditions but not as efficiently as conductors.
Temperature Dependence: As temperature increases, the number of free charge carriers (electrons and holes) in semiconductors increases, lowering their resistivity.
Band Gap: The energy gap between the valence band and conduction band is small in semiconductors, allowing electrons to move to the conduction band when sufficient energy is provided.
Types of Semiconductors
There are 2 types of Semiconductors:
Intrinsic Semiconductors
Extrinsic Semiconductors
Intrinsic Semiconductors
Intrinsic Semiconductors are pure materials without any impurities. The most common intrinsic semiconductors are silicon and germanium. At absolute zero temperature, they act as insulators, but as temperature increases, some electrons move to the conduction band, allowing current to flow.
Energy Band Diagram of Intrinsic Semiconductor
The energy band diagram of an intrinsic semiconductor is shown below.
In intrinsic semiconductors, current is carried by both free electrons and holes. The total current is the sum of the electron current (Ie), caused by thermally excited electrons, and the hole current (Ih).
Thus, the total current (I) is given by:
I = Ie + Ih
At a finite temperature, the likelihood of electrons being in the conduction band in an intrinsic semiconductor decreases exponentially as the band gap (Eg) increases.
The equation describing this is:
n = n0 * e^(-Eg / (2 * Kb * T))
Where:
Eg is the energy band gap,
Kb is Boltzmann's constant,
T is the temperature in Kelvin,
n0 is the number of free electrons at absolute zero
Extrinsic Semiconductors
When small amounts of impurities are added to intrinsic semiconductors, the conductivity is enhanced. This process is known as doping. Depending on the type of impurity added, extrinsic semiconductors are classified into two types:
N-Type Semiconductors: Doped with pentavalent impurities (e.g., phosphorus), increasing the number of free electrons.
P-Type Semiconductors: Doped with trivalent impurities (e.g., boron), creating more holes that act as positive charge carriers.
N-Type Semiconductor
An N-type semiconductor primarily conducts due to electrons. The crystal remains electrically neutral overall, but there is a significant difference in the number of electrons and holes.
In N-type semiconductors:
The majority carriers are electrons, and the minority carriers are holes.
When a pure semiconductor, like silicon or germanium, is doped with a pentavalent impurity (e.g., phosphorus, arsenic, antimony, or bismuth), four out of five valence electrons from the dopant atom bond with four electrons of the semiconductor atoms.
The fifth electron of the dopant atom is free to move, thus contributing to the conduction process. This free electron is provided by the donor atom.
The increase in free electrons causes more negative charge carriers, making it an N-type semiconductor.
Although the crystal is neutral overall, the donor atom becomes a positive ion, and the majority carriers are free electrons, while the minority carriers are holes.
P-Type Semiconductor
A P-type semiconductor primarily conducts due to holes. Similar to the N-type semiconductor, the crystal remains neutral, but the nature of the charge carriers is different.
In P-type semiconductors:
The majority carriers are holes, and the minority carriers are electrons.
When a pure semiconductor is doped with a trivalent impurity (such as boron, aluminium, indium, or gallium), the impurity's three valence electrons bond with three of the semiconductor's four valence electrons.
This bonding leaves an electron hole, creating a vacancy for an electron to move into. These acceptor atoms are ready to accept electrons from neighbouring atoms.
As the number of acceptor impurities increases, more holes are created, leading to an increase in positive charge carriers.
The crystal remains neutral, but the acceptor atoms become immobile negative ions, and the majority carriers are holes, while the minority carriers are electrons.
Difference between Intrinsic and Extrinsic Semiconductors
Applications of Semiconductors
Semiconductors are at the heart of modern electronics, with their applications spanning various fields:
Microchips: Found in computers, mobile phones, and other electronic devices.
Transistors: Used as switches and amplifiers in electronic circuits.
Solar Cells: Gallium arsenide is used to convert sunlight into electricity.
LEDs and Lasers: Used in displays, lighting, and optical communication.
Temperature Sensors: Semiconductor materials are commonly used to measure temperature variations in devices.
Self-driving Cars and 3D Printing: Semiconductor-based sensors and chips are key in advanced technology like self-driving vehicles and modern manufacturing processes.
Importance of Semiconductors
Semiconductors play a pivotal role in modern electronics due to their:
Compact size: Semiconductor devices are smaller and more efficient than other alternatives.
Lower power consumption: They use less energy, making them ideal for mobile devices.
High durability: Semiconductor devices are resistant to environmental factors and last longer.
Versatility: They can be used in a wide range of applications, from household electronics to space exploration.
Conclusion
Semiconductors are foundational to modern technology. From transistors to solar cells, they allow us to harness electrical energy, communicate, and power devices efficiently. Understanding the basic principles of semiconductor physics helps us appreciate their role in electronics and innovation.
FAQs on Semiconductor
1. What is a semiconductor and why is it essential in modern electronics?
A semiconductor is a material whose electrical conductivity falls between that of conductors and insulators. Its ability to control electricity precisely makes it essential for devices such as computers, mobile phones, and solar cells, where controlled current flow is required.
2. How do intrinsic and extrinsic semiconductors differ, as per the CBSE Class 12 Physics syllabus?
Intrinsic semiconductors are pure forms (like silicon, germanium) with low conductivity, while extrinsic semiconductors have added impurities (doping) to increase their conductivity. In extrinsic semiconductors, the type of impurity determines whether electrons (N-type) or holes (P-type) are the majority carriers.
3. Why are N-type and P-type semiconductors named so, and what role do they play?
N-type semiconductors have an excess of electrons as majority charge carriers, created by doping with elements having more valence electrons (like phosphorus). P-type semiconductors have more holes, formed by doping with elements having fewer valence electrons (like boron). Together, these materials enable the functioning of crucial devices such as diodes and transistors.
4. What is the significance of the band gap in semiconductors?
The band gap is the energy difference between the valence band and the conduction band in a semiconductor. A small band gap allows electrons to move to the conduction band when external energy (like heat or light) is provided, giving semiconductors their unique and controllable conductivity.
5. What happens inside a semiconductor when its temperature increases?
As the temperature increases, more electrons gain enough energy to jump from the valence band to the conduction band, resulting in a higher concentration of charge carriers. This causes the conductivity of semiconductors to increase and their resistivity to decrease.
6. Can you explain the concept of holes and how their mobility compares to electrons in semiconductors?
A hole represents the absence of an electron in the valence band, acting as a positive charge carrier. In semiconductors, electrons have higher mobility than holes because they move more freely in the conduction band, whereas holes move by the displacement of electrons in the valence band, facing more resistance.
7. How does doping improve the electrical properties of semiconductors?
Doping introduces small amounts of impurity atoms into a pure semiconductor. Depending on whether the dopant has more or fewer valence electrons than the host, it increases available electrons (N-type) or holes (P-type), significantly enhancing the semiconductor's electrical conductivity and making it suitable for specific electronic applications.
8. What are some key applications of semiconductors in everyday life?
- Microchips in computers and smartphones
- Transistors and diodes in circuits
- Solar cells for energy conversion
- LEDs and laser diodes in lighting and displays
- Sensors in automobiles and temperature measurement devices
9. Why are semiconductors considered more efficient and reliable than other materials for electronic devices?
- Compact size enables the making of small, portable devices
- Lower power consumption increases energy efficiency
- Durability makes them long-lasting and stable
- Versatility allows use in a range of applications from computers to medical devices
10. How does the band theory explain the working of semiconductors?
Band theory divides energy levels in solids into bands. In semiconductors, the band gap between the valence band and conduction band is small. When external energy is supplied, electrons can cross this gap, enabling controlled conduction and making semiconductors suitable for electronic switching and amplification.
11. What is the Fermi level in the context of semiconductors, and how does it affect their electrical behavior?
The Fermi level is the energy level at which the probability of finding an electron is 50%. In semiconductors, its position determines the distribution of electrons and holes, influencing whether a material behaves as N-type or P-type and how it conducts electricity.
12. How do real-world innovations leverage the unique properties of semiconductors?
Innovations such as microchips for computation, solar cells for renewable energy, and sensors in automation all rely on the ability of semiconductors to switch, amplify, and detect signals efficiently. This adaptability has driven advancements in technology, making devices smarter and more energy-efficient.























