




Introduction: Organic Chemistry - Some Basic Principles and Techniques
The term "Organic Chemistry" was coined by Berzelius in 1807 to denote the study of substances obtained from natural sources. This was founded on the vitalism idea, which claimed that all living systems have a 'vital force' that non-living systems do not. Because organic molecules are produced from living natural sources, they were assumed to be fundamentally different from inorganic compounds.
All of the molecules produced above, however, shared the presence of carbon. Carbon has a unique feature called catenation. Carbon can form long chains and rings with other carbon atoms, as well as atoms from many other elements in the periodic table (self-catenation) (cross-catenation). As a result, carbon can be used to make a variety of compounds. This is the organic chemistry chapters for NEET and JEE.
The vital force is most likely explained by the fact that most life-giving and life-sustaining tasks are done by carbon compounds, such as proteins forming human tissues and skin, haemoglobin allowing respiration, DNA/RNA carrying information in our genes, and so on.
Important Topics for Organic Chemistry - Some Basic Principles And Techniques
Sigma Bond
Pi Bond
Chemical Formulas
Hybridisation
Resonance
Inductive Effect
Important Concepts for Organic Chemistry Some Basic Principles And Techniques Notes for NEET
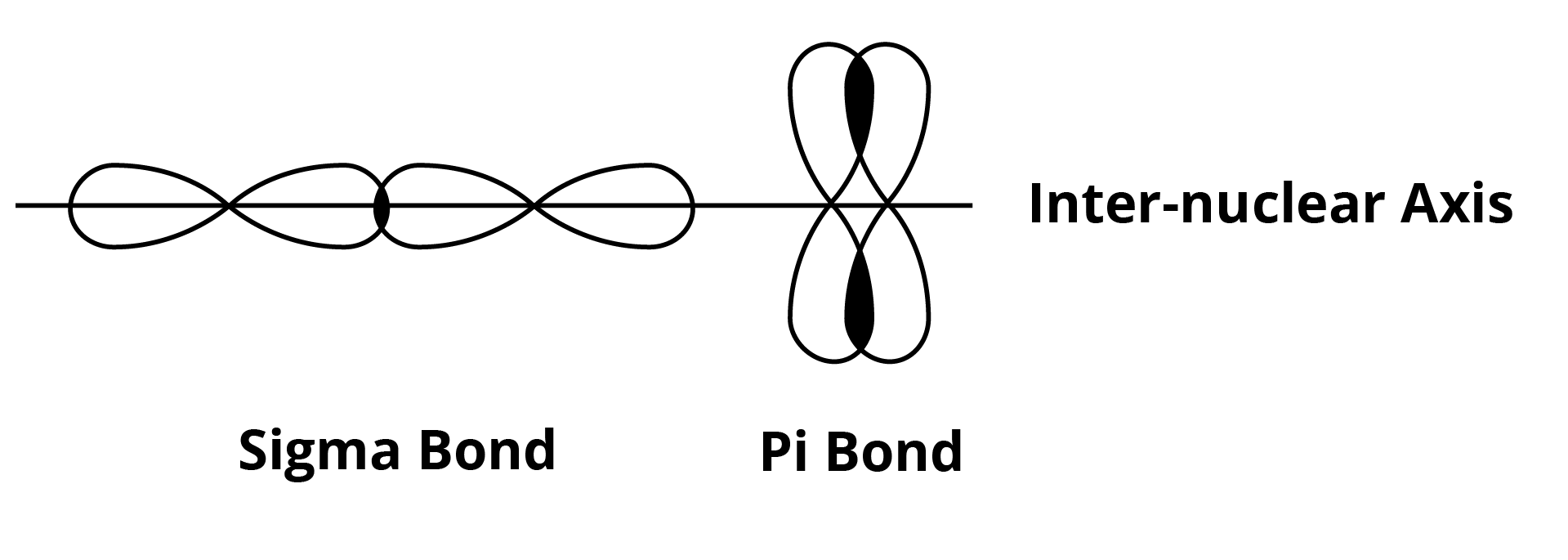
Bonding Between Atoms: Sigma and Pi Bond
Bonds are often generated and broken in a succession of separate processes before being transformed into products.
The mechanism of the reaction then becomes a step-by-step description of the entire process.
The overlapping of a sigma bond is axial or head on whereas a pi bond is parallel, lateral or sideways. The images are given below:
Chemical Formulas
Structural Formulas: Organic chemists represent organic substances with a variety of formulas.
Complete Formulas: In complete formulations, Lewis structures are utilised to represent all bond pairs of electrons as a dash (–). A pair of dots represents a single pair of electrons.
Condensed Formulas: Individual bonds are not included in condensed formulations. Each core atom, as well as the atoms that are connected to it, is represented.
Line-Angle Formulas: Skeletal structures or stick figures are other names for these.
a. For cyclic and, on rare instances, non-cyclic compounds, line-angle formulas are commonly used.
b. Carbon atoms are presumed to be present where two lines connect or where a line begins or ends, because lines symbolise bonds.
c. Most of the time, hydrogens are implied in the diagrams.
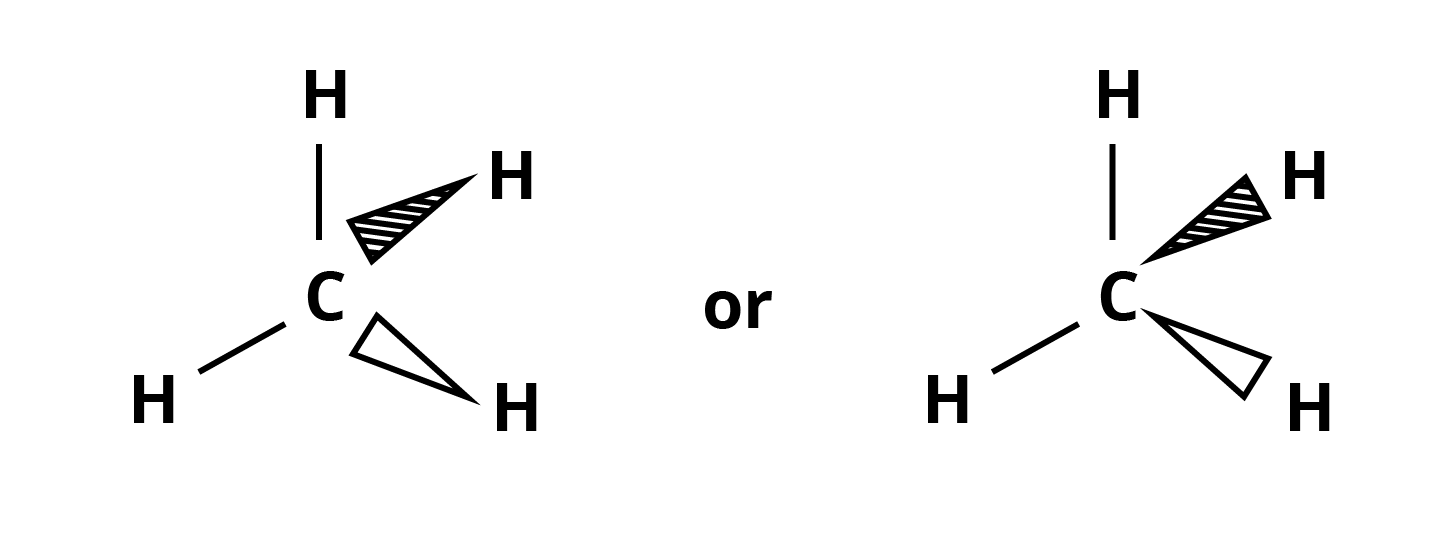
Tetrahedral representation: In general, this is a three-dimensional (3-D) representation of molecules.
a. A dashed Wedge or a solid Wedge denotes bonds that project behind the plane (away from the observer) or out of the plane (towards the observer).
b. A normal line (—) represents bonds in the plane of paper.
Degrees of Carbon and Hybridisation
The number of carbons connected to the carbon under investigation is measured in degrees of carbon.
Hybridisation is a process in which two or more atomic orbitals with similar energies in the valence shell of an atom (central atom of a molecule or ion) combine to form new degenerate orbitals known as hybrid orbitals.
As the fraction of s-character grows, the size of the hybrid orbital shrinks. As a result, the hybrid orbitals size is sp3 > sp2 > sp.
The electronegativity of the hybrid orbital grows as the percentage of s-character increases. As a result, sp > sp2 > sp3 is the electronegativity of the hybrid orbital.
Breaking of Bonds
The covalent bond is an important binding in organic chemistry for studying reactions.
As a result, we look at ways to break a covalent connection. Hence, the two ways of breaking of bonds are:
(a) Homolytic Fission
(b) Fission by Heterolysis
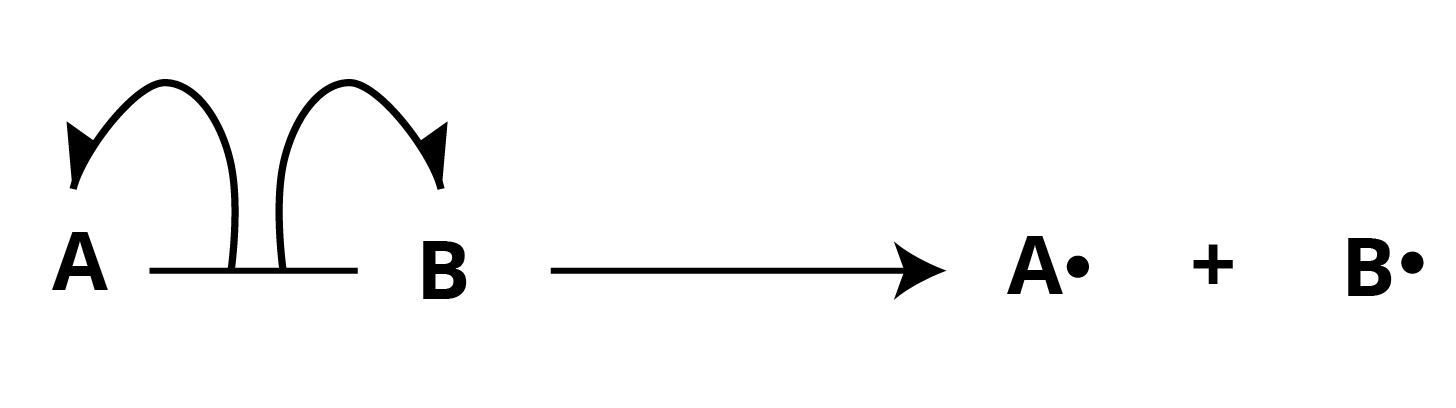
Homolytic Cleavage or Homolytic Fission:
In this type of bond breakdown, each atom loses one electron, resulting in the production of highly reactive species known as radicals (or free radicals).
Bond breaking is symbolised by two half-headed or fish hook arrows. A half-headed arrow represents the movement of one electron. Radicals are unusual electron species that are both positive and negative.
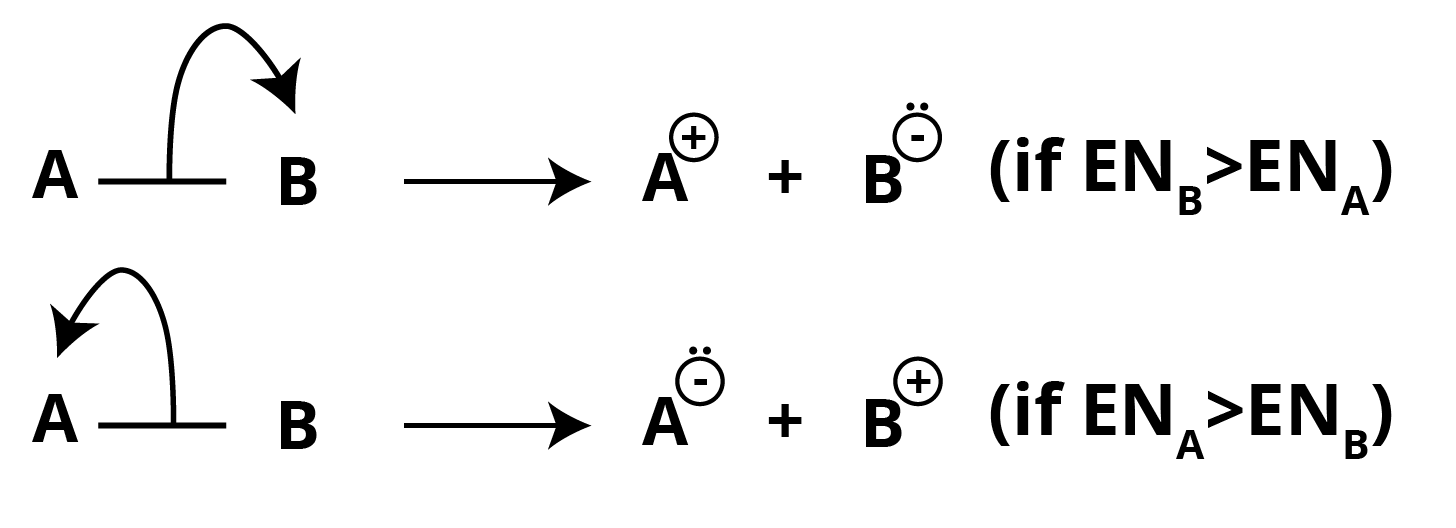
Heterolytic Fission or Homolytic Cleavage
In this sort of covalent bond breakage, the shared pair of electrons is transferred to the more electronegative component.
A cation and an anion are generated as a result of this fission (ion-pair).
The link breaking is symbolised as a full-headed arrow. The movement of two electrons is depicted by a full-headed arrow.
The movement of electrons in organic chemistry is always depicted by curving arrows, either half-headed or full-headed.
Inductive Effect
When two dissimilar atoms make a covalent link, the electron pair that forms the sigma bond is never evenly distributed between the two atoms, but is pushed slightly toward the more electronegative species.
There are three different sorts of groups/atoms that can be attached to carbon, as shown in the diagram.
Despite the fact that C is more electronegative than H, the difference in electronegativity is minimal, and the bond is considered nonpolar.
The dipole moment is precisely proportional to the inductive effect, which is a permanent effect. Because electrons are only displaced via sigma bonds, this is a minimal effect.
An example of how the inductive effect works has been highlighted, which is self-explanatory.
Groups That Donate Electrons and Those That Withdraw Electrons
A single atom or a collection of atoms can create the inductive effect. The relative inductive effects are calculated using hydrogen as a reference.
Electron-donating groups (EDG) or electron-releasing groups (ERG) give electrons to the carbon chain and are considered to have a +I effect.
Electron-withdrawing groups (EWG) are those that pull electrons out of the carbon chain, causing the –I effect.
Resonance
Simple Lewis structures are frequently employed to represent molecules, although certain molecules are too complex to be described by a single Lewis structure. As a result, the term "resonance" came into being.
The term "resonance" refers to the delocalization of electrons (usually pi electrons).
Mesomeric Effect
The electrons in the system transmit the permanent polarisation generated by a group conjugated with a pi bond or a set of alternative bonds, resulting in a distinct electron distribution in the unsaturated chain.
The redistribution of electrons in unsaturated compounds conjugated with electron-releasing or electron-drawing groups is known as the Mesomeric Effect or Resonance Effect (or atoms). This impact is lasting, as indicated by the dipole moment.
The M or R effect refers to groups that use resonance to release or withdraw electrons.
All of the groups listed share a lone pair that the atom attached to the conjugated system has to donate. As a result, it is possible to represent a generic representation.
The atom connected to the conjugated system has a bond with another more electronegative atom that either withdraws electrons or directly has a positive charge on it. As a result, Y=Z (ENZ > ENY) can be used to express a general representation.
Applications of Mesomeric Effect
The charge-separated structure of carboxylic acid is less stable than the charge-delocalized carboxylate ion due to its resonant structure. As a result, carboxylic acid loses proton (H) quickly and forms the carboxylate ion.
In phenol, resonance produces charge separation, which speeds up the ionisation process and leads to the creation of the phenoxide ion, which is stabilised by charge delocalization.
The stronger the link between C and Z, the more difficult it is for a nucleophile to break a bond, and thus the lower the reactivity. The following is the reactivity order of carboxylic acid derivatives when it comes to nucleophilic acyl substitution: Acid Anhydride > Ester > Amide > Acyl Chloride.
EWG raises acidic strength while lowering basic strength. ERG reduces acidic strength while increasing acidic strength.
Applications of Organic Chemistry in Our Daily Lives:
Organic chemistry has a profound impact on various aspects of our daily lives. Here are some more examples -
Cosmetics and Personal Care Products: Organic chemistry is behind the creation of cosmetics and personal care items we use daily, such as shampoos, soaps, and lotions. It helps formulate these products with ingredients that cleanse, moisturize, and enhance our appearance.
Cleaning Agents: Many household cleaning products, like detergents, disinfectants, and stain removers, are based on organic chemistry. These compounds break down and remove stains, dirt, and germs, making our surroundings clean and hygienic.
Fuels: Organic chemistry is intimately linked to the production of fuels that power our vehicles and homes. Gasoline and diesel, derived from organic compounds, keep our transportation systems running smoothly. Moreover, organic chemistry is pivotal in the development of sustainable biofuels, reducing our carbon footprint.
Plastics: Plastics, found in a wide range of products from packaging materials to toys, are made from organic compounds. Understanding the chemistry behind these materials has led to innovations in recyclable and biodegradable plastics, addressing environmental concerns.
Agriculture: Organic chemistry is crucial in the development of pesticides and fertilizers that protect crops and enhance agricultural yields. It also aids in the study of plant compounds and natural products, contributing to the field of herbal medicine and organic farming practices.
Solved Examples from the Chapter
Question 1: What is each carbon atom's hybridisation state in the compound: CH2=C=O?
Solution:
Let the carbon of the methyl group, CH2 in the given compound be C1 and the carbonyl carbon i.e. carbon of C=O, be C2.
Here, C1 is sp2 hybridised and C2 is sp hybridised.
Key points to remember: Typically the carbon having more single bonds is sp2 hybridised and the ones surrounding the pi bonds are sp hybridised.
Question 2: In the given molecule, identify the sigma and pi bonds: HCONHCH3.
Solution:
The structure of the given compound is given by the structural formula given below:
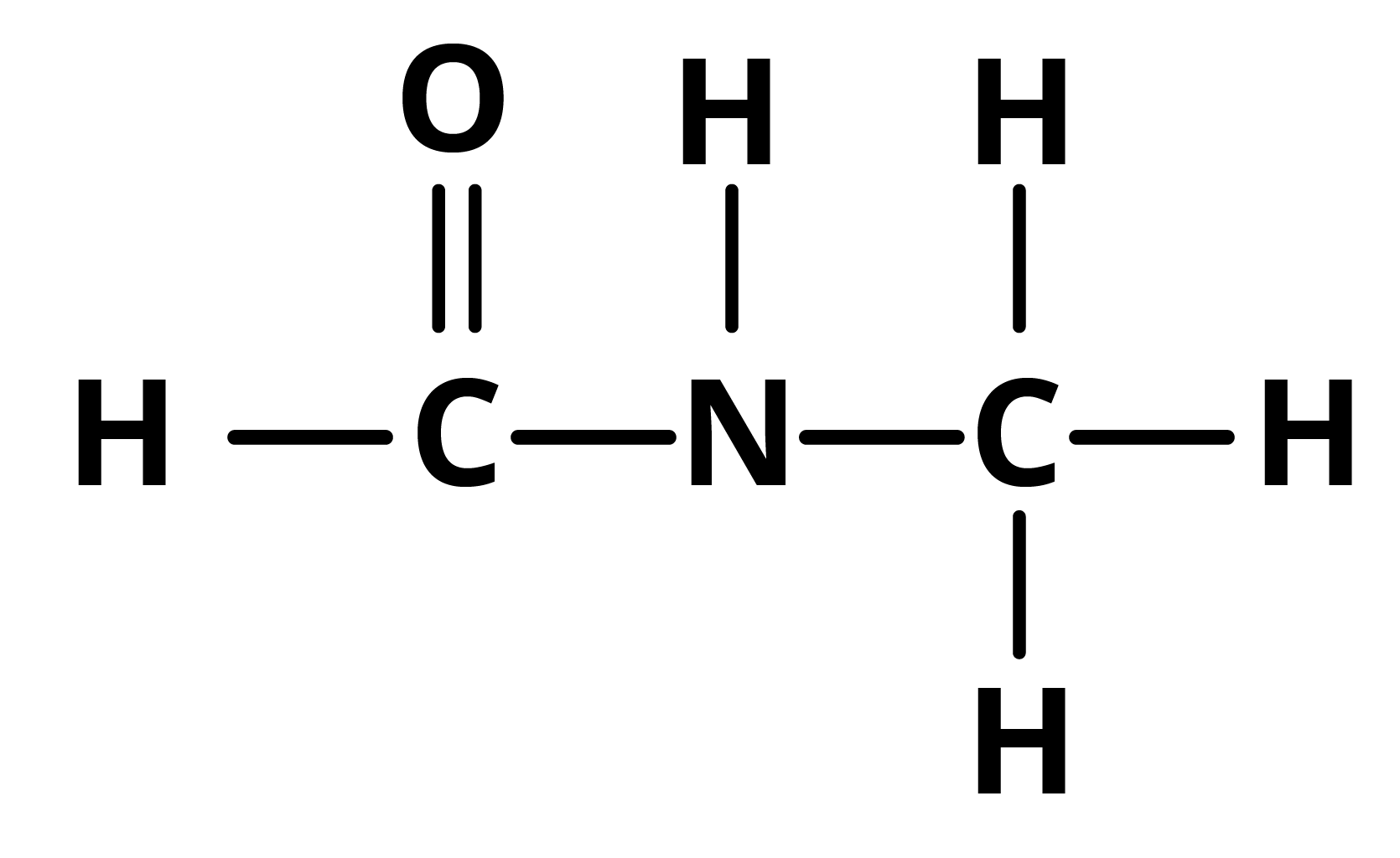
In the above compound, two C-N bonds are sigma bonds, four C-H bonds are sigma bonds, one N-H bond is a sigma bond, and one C=O bond is a pi bond.
Key points to remember: All sigma bonds are single bonds, whereas all double and triple bonds are pi bonds.
Solved Examples from Previous Year Question Papers
Question 1: The most basic compound among the following is:-
Acetanilide
Benzylamine
p-Nitroaniline
Aniline
Solution:
The resonance of lone pair electrons is inversely related to basicity. Benzylamine is a more basic substance.
In benzylamine, the electron pairs are not involved in resonance.
The lone pair of electrons on the N atom on the ring is delocalized in other amines.
The neighbouring CO group causes delocalization of the lone pair of electrons on the N atom in acetanilide.
As a result, option (b) is the correct answer.
Question 2: The general formula CnH2nO2 could be for open chain
carboxylic acids
diols
dialdehydes
diketones
Solution:
Open chain carboxylic acid or ester could be represented by the generic formula CnH2nO2.
As a result, option (a) is the correct answer.
Question 3: Which one the following does not have sp2 hybridised carbon?
Acetone
Acetamide
Acetonitrile
Acetic acid
Solution:
Only sp3 and sp hybridised carbon atoms are found in acetonitrile.
As a result, option (c) is the correct answer.
Practice Questions
Question 1: CH3CHO's IUPAC name is
Acetaldehyde
Methyl aldehyde
Ethanol
Ethanal
Answer: (d) Ethanal
Question 2: The functional group -OH is found in which of the following compounds?
1, 2-ethanediol
2-butanone
Nitrobenzene
Ethanal
Answer: (a) 1, 2-ethanediol
Importance of Learning Chapter - Organic Chemistry - Some Basic Principles and Techniques for NEET
Organic Chemistry, particularly the chapter "Some Basic Principles and Techniques," holds immense significance for NEET (National Eligibility cum Entrance Test) aspirants. This chapter forms the foundation for understanding the intricacies of organic compounds and their reactions. Here's why it's crucial:
Fundamental Concepts: This chapter introduces students to fundamental concepts like structural representations, isomerism, and nomenclature. A strong grasp of these concepts is essential for recognizing and differentiating between various organic compounds, a skill vital for NEET.
Reactivity Patterns: Organic Chemistry helps students comprehend the reactivity patterns of different functional groups. It provides insights into how and why organic compounds react the way they do. This knowledge is invaluable when predicting and understanding chemical reactions in biology and medicine.
Practical Applications: Organic Chemistry is not just theoretical; it has practical applications in pharmacology, medicine, and biochemistry. Understanding the basics is essential for future doctors and healthcare professionals to grasp drug design and bioorganic processes.
Solving Complex Problems: NEET exams often present complex questions related to organic compounds and reactions. Those well-versed in this chapter can tackle these questions with confidence, contributing to higher scores.
Building Blocks: Organic Chemistry is the building block for other advanced topics in chemistry. Whether it's biochemistry, medicinal chemistry, or chemical engineering, this chapter lays the groundwork for more specialized studies.
Laboratory Skills: Understanding the basic techniques in this chapter is essential for any student entering the field of science. Techniques like purification, separation, and identification are not only valuable for the NEET exam but also for future laboratory work in research or clinical settings.
Conclusion
We got over some fundamental ideas in the structure and reactivity of organic compounds created by covalent bonding in this chapter. Organic compounds' covalent bonding nature is described in terms of orbitals hybridization, with carbon having sp2, sp, and sp3 hybridised orbitals. Ethane, ethyne, and methane are examples of sp, sp2, and sp3 hybridised carbons. Based on this understanding, the shape of these compounds is simple to comprehend. This is important for organic chemistry chapters for neet notes.
NEET Chapter Page: Organic Chemistry - Some Basic Principles and Techniques

 Share
ShareFAQs on NEET Chapter Page: Organic Chemistry - Some Basic Principles and Techniques
1. What is the basic principle of organic chemistry?
About 200 years ago, organic chemistry emerged as a science. Organic and inorganic compounds were separated into two categories by the late eighteenth century. Plants and animals were used to create organic chemicals. Organic molecules were harder to work with in the lab and disintegrate quickly. All organic compounds contain carbon, thus studying organic chemistry entails looking at carbon-based molecules to understand what types of reactions they go through and how they're put together. As a result, organic chemistry is the branch of chemistry concerned with carbon compounds.
2. What is the difference between electrophiles and nucleophiles?
Chemical species that donate or take electrons to establish a new chemical bond are known as electrophiles and nucleophiles. A nucleophile is a chemical species that donates an electron pair to establish a chemical bond in response to a stimulus. An electrophile is a molecule, ion, or atom that is lacking in electrons in some way. A nucleophile is usually negatively or neutrally charged, with only a few donable electrons. Examples include H2O, -OMe, and -OtBu. The electron-rich is a nucleophile in general. Electrophiles are positively charged or neutral organisms with vacant orbitals that are attracted to an electron-rich centre.
3. What are organic chemistry's characteristics?
Physical properties of organic compounds of interest often contain both quantitative and qualitative characteristics. Melting point, boiling point, and index of refraction are all examples of quantitative data. Aroma, consistency, solubility, and colour are examples of qualitative qualities.
4. What is the full form of CTC in NEET organic chemistry?
Carbon tetrachloride, widely used in factories as a cleaning agent, is a chemical compound with the formula CCl4. It is a colorless liquid with a sweet odor that can be detected at low concentrations. In the power industry, CTC is used primarily to clean the contacts of electrical equipment.
5. How to study organic chemistry some basic principles and techniques for NEET?
Organic chemistry is a challenging subject, but it is also very rewarding. Here are some tips on how to study organic chemistry:
Understand the basics: Make sure you have a solid understanding of the basic concepts of organic chemistry, such as the structure of organic molecules, functional groups, and bonding. You can use Vedantu’s resources for a better understanding.
Practice drawing and naming organic molecules: This will help you to visualize and understand the structures of organic molecules.
Learn the mechanisms of organic reactions: This will help you to understand how organic reactions work and to predict the products of organic reactions.
Solve organic chemistry problems: This is the best way to test your understanding of organic chemistry concepts and mechanisms.




















 Watch Video
Watch Video


