
Which of the following is most stable?
A. \[\text{S}{{\text{n}}^{\text{2+}}}\]
B. \[\text{G}{{\text{e}}^{\text{2+}}}\]
C. \[\text{S}{{\text{i}}^{\text{2+}}}\]
D. \[\text{P}{{\text{b}}^{\text{2+}}}\]
Answer
233.1k+ views
Hint: Here the stability of oxidation states comes into play, so we should try to check the stabilisation of multiple oxidation states of the carbon family and also the various factors which influences it.
Complete step by step solution: The given compounds belong to the carbon family, which shows a covalency of 4.
-So,the common oxidation states exhibited by this family is +2 and +4.
-Now,the tetravalency is explained by the shifting of one of the paired electrons of ns orbital which occupies the np orbital during the excited state, thus giving us an oxidation state of +4.
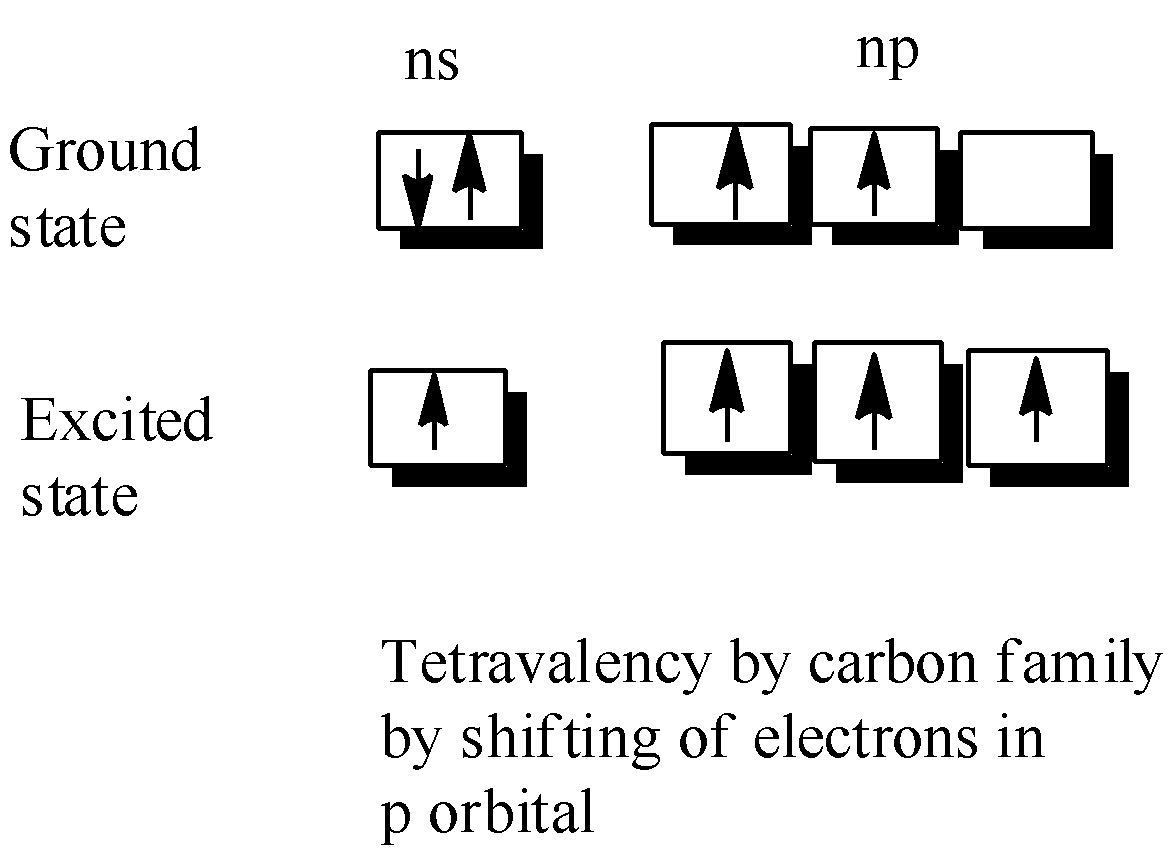
-But in heavier members,we usually notice that the tendency to show +2 oxidation state increases in the sequence Ge-Now,here a common doubt arises what reasons influence this change in behaviour of the carbon family
So, we will try to understand this by a condition known as inert pair effect.
- First, we will try to understand two terms related to inert pair effect:
-Effective nuclear charge:It is generally defined as the net positive charge experienced by an atom inside it’s nucleus.
-Shielding effect :Outer electrons experience a nuclear attraction from the positive charges of the nucleus and face repulsion from the similarly charged inner electrons. So, due to immense repulsion by the inner shell electrons,the valence electrons experience reduction in attraction from the nucleus,and thus the reduced attraction between the nucleus and electrons is referred to as shielding effect.
-Now in inert pair effect, the the \[\text{n}{{\text{s}}^{\text{2}}}\] electrons of the outermost orbit,remains unshared in some of the compounds as the energy required to unpair the electrons in s orbitals is quite high,as the inner electrons experience high nuclear charge,due to the poor shielding effect of ‘s’,’d’,’f’,orbitals and thus do not participate in bonding.Owing to this,only the valence orbitals participate in bonding and thus this is known as inert pair effect.
-Due to this effect,down a group,the tendency of forming \[{{\text{M}}^{\text{2+}}}\] ions keeps on increasing from Ge to Pb and thus the following stability order in increasing order of atomic number is followed:
-\[\text{S}{{\text{i}}^{\text{2+}}}\text{G}{{\text{e}}^{\text{2+}}}\text{S}{{\text{n}}^{\text{2+}}}\text{P}{{\text{b}}^{\text{2+}}}\]
-Thus in the given question we can infer that \[\text{P}{{\text{b}}^{\text{2+}}}\] is the most stable.And the correct answer is option D.
Additional Information:The relative stabilities of these two oxidation states varies down the group. Carbon and Silicon mostly show +4 oxidation state , whereas Germanium forms stable compounds in +4 state.Tin forms compounds in both oxidation states,and lead compounds in +2 states are more stable.
Note: We should remember that as +2 oxidation state is more stable then +4 in going down in this carbon family, and so they act accordingly as oxidising and reducing agents in their variable oxidation states.
Complete step by step solution: The given compounds belong to the carbon family, which shows a covalency of 4.
-So,the common oxidation states exhibited by this family is +2 and +4.
-Now,the tetravalency is explained by the shifting of one of the paired electrons of ns orbital which occupies the np orbital during the excited state, thus giving us an oxidation state of +4.

-But in heavier members,we usually notice that the tendency to show +2 oxidation state increases in the sequence Ge
So, we will try to understand this by a condition known as inert pair effect.
- First, we will try to understand two terms related to inert pair effect:
-Effective nuclear charge:It is generally defined as the net positive charge experienced by an atom inside it’s nucleus.
-Shielding effect :Outer electrons experience a nuclear attraction from the positive charges of the nucleus and face repulsion from the similarly charged inner electrons. So, due to immense repulsion by the inner shell electrons,the valence electrons experience reduction in attraction from the nucleus,and thus the reduced attraction between the nucleus and electrons is referred to as shielding effect.
-Now in inert pair effect, the the \[\text{n}{{\text{s}}^{\text{2}}}\] electrons of the outermost orbit,remains unshared in some of the compounds as the energy required to unpair the electrons in s orbitals is quite high,as the inner electrons experience high nuclear charge,due to the poor shielding effect of ‘s’,’d’,’f’,orbitals and thus do not participate in bonding.Owing to this,only the valence orbitals participate in bonding and thus this is known as inert pair effect.
-Due to this effect,down a group,the tendency of forming \[{{\text{M}}^{\text{2+}}}\] ions keeps on increasing from Ge to Pb and thus the following stability order in increasing order of atomic number is followed:
-\[\text{S}{{\text{i}}^{\text{2+}}}\text{G}{{\text{e}}^{\text{2+}}}\text{S}{{\text{n}}^{\text{2+}}}\text{P}{{\text{b}}^{\text{2+}}}\]
-Thus in the given question we can infer that \[\text{P}{{\text{b}}^{\text{2+}}}\] is the most stable.And the correct answer is option D.
Additional Information:The relative stabilities of these two oxidation states varies down the group. Carbon and Silicon mostly show +4 oxidation state , whereas Germanium forms stable compounds in +4 state.Tin forms compounds in both oxidation states,and lead compounds in +2 states are more stable.
Note: We should remember that as +2 oxidation state is more stable then +4 in going down in this carbon family, and so they act accordingly as oxidising and reducing agents in their variable oxidation states.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

Hydrocarbons Class 11 Chemistry Chapter 9 CBSE Notes - 2025-26

Thermodynamics Class 11 Chemistry Chapter 5 CBSE Notes - 2025-26

Equilibrium Class 11 Chemistry Chapter 6 CBSE Notes - 2025-26

Organic Chemistry Some Basic Principles And Techniques Class 11 Chemistry Chapter 8 CBSE Notes - 2025-26

NCERT Solutions For Class 11 Chemistry Chapter 7 Redox Reactions (2025-26)




