
What would be the product formed when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether?
A . 1-Bromocyclobutane
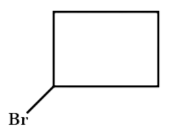
B . 1 - Chlorocyclobutane
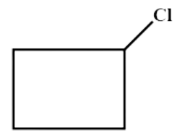
C . Cyclobutane

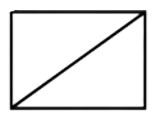
D . Bicyclo ( 1 , 1 , 0 ) ) butane
Answer
231.6k+ views
Hint: In this question, intramolecular Wurtz reaction is taking place. With the help of radical species R, the Wurtz reaction entails the transfer of halogen and metal in order to create a carbon-carbon bond that results from a nucleophilic substitution process. The process involves reacting alkyl halides with metallic sodium in the presence of dry ether to produce higher alkanes.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
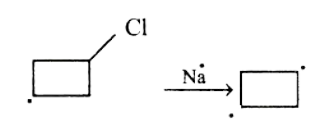
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
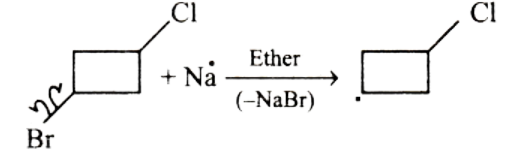
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.
The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:
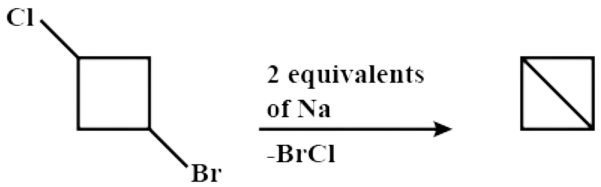
Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Wurtz reaction equation:
R-X + 2Na + X-R→ R–R + 2NaX, where X = halogen (Cl, Br, I)
Complete step by step solution:
Given that 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether.
In the first step, Na will attack the Bromine-Carbon bond and a carbanion will be formed on the chlorocyclobutane.
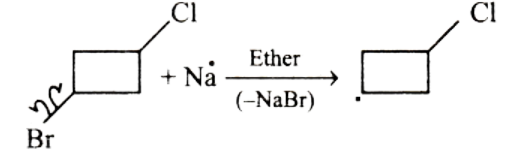
After the formation of carbanion, Na will attack the Chlorine-Carbon bond and another carbanion will be formed on the chlorocyclobutane.

The formation of two carbanions on a 4-membered ring is very unstable and hence the two carbanions join to form a bond, and thus form Bicyclo ( 1 , 1 , 0 ) butane

Hence, the overall chemical reaction when 1−bromo−3−chlorocyclobutane reacts with two equivalents of metallic sodium in the ether is:

Thus, Option (D) is correct.
Note: A BrCl molecule is removed in this intramolecular Wurtz reaction, and a Carbon-Carbon bond is created, producing a bicyclic compound. The order of reactivity of alkyl halides in Wurtz reaction is R-I(alkyl iodide) >R-Br(alkyl bromide) >R-Cl(alkyl chloride), because Carbon-Bromine bond is weaker than Carbon-Chlorine bond. Hence. Wurtz reaction will take place with C-Br bond in the first step.
Recently Updated Pages
JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

Electricity and Magnetism Explained: Key Concepts & Applications

Algebra Made Easy: Step-by-Step Guide for Students

JEE Energetics Important Concepts and Tips for Exam Preparation

Trending doubts
JEE Main 2026: Admit Card Out, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




