
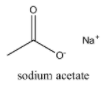
Sodium acetate on heating with soda lime produce:
(A) $C{{H}_{4}}$
(B) ${{C}_{2}}{{H}_{6}}$
(C) ${{C}_{3}}{{H}_{8}}$
(D) ${{C}_{2}}{{H}_{4}}$
Answer
527.5k+ views
Hint: Sodium salt of carboxylic acid on heating with soda lime produces alkane with one less carbon atom than carboxylic acid. Soda-lime is a mixture of sodium hydroxide and calcium oxide. Acetic acid has two carbon atoms. It is a decarboxylation reaction as there is the elimination of carbon dioxide from a carboxylic acid.
Complete step by step solution:
-Decarboxylation is a method to prepare alkane using sodium salt of carboxylic acid.
-This method can be used when one less carbon atom than carboxylic acid is required or when the number of carbon atoms is to be reduced by one.
-When sodium salt of the carboxylic acid is treated with soda lime which is a mixture of sodium hydroxide and calcium oxide, carbon dioxide is eliminated so an alkane is produced with one less carbon atom than carboxylic acid and sodium bicarbonate is produced as a by-product.
-Calcium oxide is used so Sodium hydroxide can be easily handled. Sodium hydroxide is highly hygroscopic and easily forms concentrated sodium hydroxide when exposed to air. Soda-lime does not absorb moisture easily.
-Soda-lime has the ability to absorb carbon dioxide. When carbon dioxide is eliminated from carboxylic acid, it reacts with sodium hydroxide to form sodium carbonate.
\[C{{H}_{3}}COONa+NaOH\xrightarrow{CaO,\Delta }C{{H}_{4}}+N{{a}_{2}}C{{O}_{3}}\]
When sodium acetate on heating with soda lime, methane is produced.
Sodium acetate on heating with soda lime produce: $C{{H}_{4}}$, which is option (A)
Note: In decarboxylation, the sodium salt of carboxylic acid on heating with soda lime produces alkane with one less carbon atom than a carboxylic acid. In decarboxylation, the product is alkane having one less carbon atom than a carboxylic acid. If ethane is to be prepared, then sodium propionate should be used. IF carboxylic acid has n number of carbon atoms, then alkane produced will have (n-1) carbon atoms.
Complete step by step solution:
-Decarboxylation is a method to prepare alkane using sodium salt of carboxylic acid.
-This method can be used when one less carbon atom than carboxylic acid is required or when the number of carbon atoms is to be reduced by one.
-When sodium salt of the carboxylic acid is treated with soda lime which is a mixture of sodium hydroxide and calcium oxide, carbon dioxide is eliminated so an alkane is produced with one less carbon atom than carboxylic acid and sodium bicarbonate is produced as a by-product.
-Calcium oxide is used so Sodium hydroxide can be easily handled. Sodium hydroxide is highly hygroscopic and easily forms concentrated sodium hydroxide when exposed to air. Soda-lime does not absorb moisture easily.
-Soda-lime has the ability to absorb carbon dioxide. When carbon dioxide is eliminated from carboxylic acid, it reacts with sodium hydroxide to form sodium carbonate.

\[C{{H}_{3}}COONa+NaOH\xrightarrow{CaO,\Delta }C{{H}_{4}}+N{{a}_{2}}C{{O}_{3}}\]
When sodium acetate on heating with soda lime, methane is produced.
Sodium acetate on heating with soda lime produce: $C{{H}_{4}}$, which is option (A)
Note: In decarboxylation, the sodium salt of carboxylic acid on heating with soda lime produces alkane with one less carbon atom than a carboxylic acid. In decarboxylation, the product is alkane having one less carbon atom than a carboxylic acid. If ethane is to be prepared, then sodium propionate should be used. IF carboxylic acid has n number of carbon atoms, then alkane produced will have (n-1) carbon atoms.
Recently Updated Pages
Types of Solutions in Chemistry: Explained Simply

JEE General Topics in Chemistry Important Concepts and Tips

JEE Extractive Metallurgy Important Concepts and Tips for Exam Preparation

JEE Amino Acids and Peptides Important Concepts and Tips for Exam Preparation

JEE Atomic Structure and Chemical Bonding important Concepts and Tips

Electricity and Magnetism Explained: Key Concepts & Applications

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




