




How Do the Shielding Effect and Effective Nuclear Charge Affect Atomic Properties?
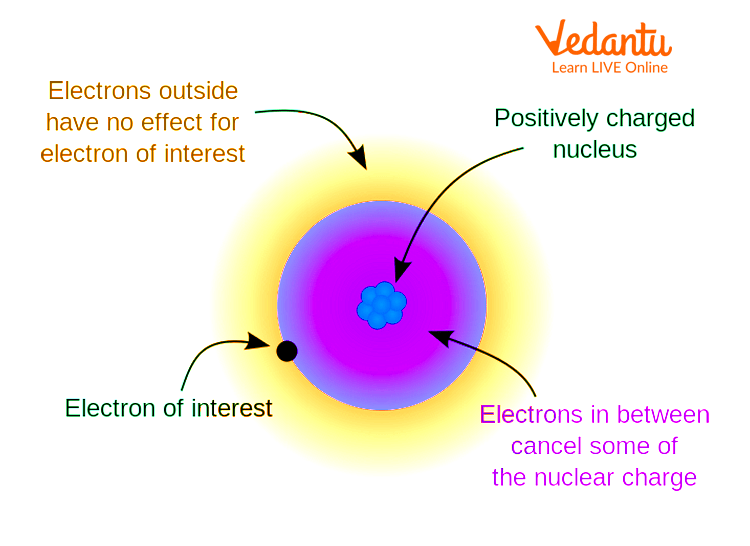
In atoms with multiple electrons, outer electrons do not feel the full attractive force of the nucleus. This happens because inner-shell electrons partially block, or "shield," the positive charge from the nucleus. This phenomenon is called the shielding effect.
Because of the shielding effect, the effective nuclear charge acting on valence electrons is less than the actual nuclear charge. The greater the number of inner electrons, the stronger the shielding.
Definition of Effective Nuclear Charge
Effective nuclear charge, denoted as $Z_{eff}$, is the net positive charge experienced by an electron within a multi-electron atom. It quantifies the real attractive force from the nucleus, after accounting for the repulsion from other electrons.
$Z_{eff}$ is crucial in understanding atomic properties such as ionization energy, atomic radius, and chemical reactivity. A higher $Z_{eff}$ means electrons are held more tightly to the nucleus.
Relation Between Shielding Effect and Effective Nuclear Charge
There is an inverse relationship between the shielding effect and effective nuclear charge. As shielding effect increases, the effective nuclear charge experienced by the outermost electrons decreases.
This relationship explains why elements lower down a group in the periodic table have larger atomic radii. Increased inner electrons enhance shielding, reducing $Z_{eff}$ for valence electrons.
For a conceptual foundation, students can refer to Atomic Structure Basics to reinforce their understanding.
Equation for Effective Nuclear Charge
The effective nuclear charge can be estimated with the equation:
$Z_{eff} = Z - S$
$Z$ is the atomic number and $S$ is the shielding constant. The value of $S$ is calculated using rules based on the electron's position.
Applying Slater’s Rules for Calculating $Z_{eff}$
Slater’s rules provide a simple way to calculate the shielding constant $S$. Electrons in different shells and subshells contribute differently to $S$.
In general, electrons in the same group provide a partial shielding value, while electrons in lower energy shells have a stronger shielding impact. This method brings accuracy to our calculation of $Z_{eff}$.
Role of Shielding and $Z_{eff}$ in Atomic Properties
The interplay between the shielding effect and effective nuclear charge determines the atomic radius, ionization energy, and electron affinity of an element.
For instance, down a group, increased shielding leads to larger atomic size, while across a period, increased $Z_{eff}$ makes the atom smaller. This core idea is vital for JEE-level questions.
Analogy: The Stadium Model
Imagine the nucleus as a bright spotlight in a stadium, shining outwards. The people standing between the light and you (representing inner electrons) block some of the light.
The farther you are, and the more people between you and the light, the dimmer the light appears. This models the shielding effect reducing the effective "brightness" (nuclear charge) you feel.
This analogy helps students visualize why outer electrons experience less effective nuclear charge than the atom’s true nuclear charge.
Characteristics of Shielding Effect
- Shielding depends on electron configuration and distance from the nucleus
- d and f electrons provide greater shielding than s and p electrons
- Shielding reduces effective nuclear charge for outer electrons
- Heavier elements show stronger shielding effect on valence shells
Comparison: Shielding Effect vs. Effective Nuclear Charge
| Shielding Effect | Effective Nuclear Charge |
|---|---|
| Reduces nuclear attraction | Measures net nuclear pull |
| Due to electron repulsion | Depends on $Z$ and $S$ |
| Increases with shell number | Increases across a period |
Sample Calculation: Effective Nuclear Charge
Let’s calculate the effective nuclear charge ($Z_{eff}$) for a 3p electron in a sulfur (S) atom.
Known values: $Z = 16$. Electron configuration: $1s^2\ 2s^2\ 2p^6\ 3s^2\ 3p^4$.
We use the Slater's rule equation:
$Z_{eff} = Z - S$
For 3p electron, same group (3s, 3p) electrons shield by 0.35 each; electrons in lower shells shield by 0.85 each.
$S = (5 \times 0.35) + (8 \times 0.85) = 1.75 + 6.8 = 8.55$
Therefore, $Z_{eff} = 16 - 8.55 = 7.45$. The 3p electron in sulfur feels a net nuclear charge of 7.45.
Practice Question for JEE
Calculate the effective nuclear charge ($Z_{eff}$) for a 2p electron in a nitrogen (N) atom using Slater’s rules. State your answer to two decimal places.
Difference Between Shielding and Screening Effect
The terms "shielding effect" and "screening effect" are often used interchangeably. Both describe how inner electrons lessen the pull of the nucleus on outer electrons in multi-electron atoms.
Screening is simply another name for shielding—there is no fundamental difference between shielding effect and screening effect at the JEE level.
Common Mistakes to Avoid in JEE Preparation
Students often confuse shielding with effective nuclear charge. Remember, shielding is the cause, while effective nuclear charge is the effect felt by electrons.
It is also common to overlook subshell differences. d and f electrons shield less effectively than s and p electrons in the same shell, and this is essential for concept clarity.
Applications in Real-World Chemistry and Physics
Understanding the shielding effect and effective nuclear charge helps predict trends in ionization energy, atomic size, and reactivity—crucial for explaining periodic properties.
These concepts are fundamental for Electrostatics Overview and play a role in semiconductor and device physics, relevant for students interested in Understanding Electronic Devices.
Test Your Understanding: Conceptual Challenge
If the shielding effect in a sodium atom increases, explain how this would impact the effective nuclear charge and the atomic radius.
Related Physics Topics
- Find more in the Electrostatics Overview section
- Review fundamentals at Atomic Structure Basics for deeper insight
- Understand current tech via Understanding Electronic Devices
- Learn statistical implications with Kinetic Theory of Gases
- Attempt simulated exams with Electrostatics Mock Test
FAQs on Understanding Shielding Effect and Effective Nuclear Charge
1. What is shielding effect in chemistry?
Shielding effect refers to the decrease in the attractive force between the nucleus and valence electrons due to the presence of inner-shell electrons. Inner electrons partially block the positive charge from the nucleus, thus shielding the outer electrons.
Key features include:
- Inner electrons reduce the pull felt by valence electrons.
- Makes it easier to remove outer electrons.
- Also known as the screening effect.
2. What is effective nuclear charge?
Effective nuclear charge (Zeff) is the net positive charge experienced by an electron in a multi-electron atom. It considers both the attractive force from the nucleus and the repulsive shielding by other electrons.
- Zeff = Z – S (where Z = atomic number, S = shielding constant)
- Higher Zeff means stronger pull on electrons.
- Key to understanding trends in atomic size and ionization energy.
3. Why does shielding effect increase down a group?
The shielding effect increases down a group because the number of inner shells increases, each adding more inner electrons between the nucleus and valence shell. These inner electrons block the attraction from the nucleus more effectively as we go down.
- New shells add more shielding.
- Leads to larger atomic radii down the group.
4. How does shielding effect influence atomic size?
A stronger shielding effect leads to a larger atomic size because the valence electrons feel a weaker pull from the nucleus and can stay farther away.
- Increased shielding = less pull on outermost electrons.
- Thus, atomic radius increases, especially down a group.
5. What factors affect the effective nuclear charge?
The main factors affecting effective nuclear charge (Zeff) include:
- Atomic number (Z): Higher Z increases Zeff.
- Shielding constant (S): More inner electrons give greater shielding and lower Zeff.
- Electron configuration and penetration of orbitals (s > p > d > f).
6. What is the order of shielding effect among s, p, d, and f orbitals?
The order of shielding effect among orbitals is:
- s > p > d > f
7. How do you calculate the effective nuclear charge for an electron?
To calculate the effective nuclear charge (Zeff) for an electron, use the formula:
- Zeff = Z - S
- Where Z = atomic number and S = number of shielding electrons.
- Simple methods like Slater’s rules help estimate S.
8. What is the difference between shielding effect and effective nuclear charge?
Shielding effect is the reduction in nuclear attraction on valence electrons due to inner electrons. Effective nuclear charge is the net force felt by an electron after shielding is taken into account.
- Shielding effect– How much nucleus pull is blocked.
- Effective nuclear charge– Actual pull felt by an electron.
- Both concepts explain trends in atomic properties.
9. Why do d and f electrons have poor shielding effect?
d and f electrons have a poor shielding effect because their shapes and penetration into the nucleus are less than s and p orbitals.
- d and f electrons are farther from the nucleus.
- They shield valence electrons less effectively.
- This influences chemical and periodic trends.
10. How does effective nuclear charge explain periodic trends?
An increase in effective nuclear charge across a period causes electrons to be held more tightly, resulting in:
- Decrease in atomic size.
- Increase in ionization enthalpy.
- Greater non-metallic character across the period.
11. What is the significance of the shielding effect in chemical bonding?
The shielding effect significantly influences chemical bonding by affecting the attraction between valence electrons and the nucleus, thereby impacting:
- Bond strength and formation
- Ionization energy
- Electronegativity values
























