
In ${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$ ion, the maximum number of bonds having the same Cr-O bond length is:
A. ${ 5 }$
B. ${ 4 }$
C. ${ 6 }$
D. ${ 3 }$
Answer
233.1k+ views
Hint: Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
Bond lengths are expressed in terms of Angstrom or picometer.
Complete step-by-step answer:
The structure of the dichromate ion is:
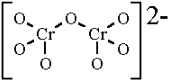
As we can see in the structure that there are six identical Cr - O bonds in the dichromate ion ${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$.
It means there are 6 identical Cr - O terminal bonds in dichromate ion. All these bonds are equivalent due to resonance.
These are the oxoanions of chromium in the oxidation state of
${ +6 }$.
Hence, In ${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$ ion, the maximum number of bonds having the same Cr-O bond length is ${ 6 }$.
Therefore, the correct option is C.
Additional Information:
1. Resonance is the delocalization of pi bonds, by shifting electrons. More the resonance structure more will be the stability.
2. These structures are similar in energy, bonding, and nonbonding pairs of electrons. Each form of structure is known as a canonical form.
3. The canonical forms of a molecule are also known as Resonating Structures.
Importance:
a) Resonance stabilizes the molecule
b) Resonance causes equality of bond lengths
c) Resonance hybrid always have the lowest energy than any canonical form
Note: The possibility to make a mistake is that you may choose option B. There are ${ 6 }$ equivalent bonds in dichromate ion, not four as all these bonds are in resonance.
Bond lengths are expressed in terms of Angstrom or picometer.
Complete step-by-step answer:
The structure of the dichromate ion is:

As we can see in the structure that there are six identical Cr - O bonds in the dichromate ion ${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$.
It means there are 6 identical Cr - O terminal bonds in dichromate ion. All these bonds are equivalent due to resonance.
These are the oxoanions of chromium in the oxidation state of
${ +6 }$.
Hence, In ${ Cr }_{ 2 }{ O }_{ 7 }^{ 2- }$ ion, the maximum number of bonds having the same Cr-O bond length is ${ 6 }$.
Therefore, the correct option is C.
Additional Information:
1. Resonance is the delocalization of pi bonds, by shifting electrons. More the resonance structure more will be the stability.
2. These structures are similar in energy, bonding, and nonbonding pairs of electrons. Each form of structure is known as a canonical form.
3. The canonical forms of a molecule are also known as Resonating Structures.
Importance:
a) Resonance stabilizes the molecule
b) Resonance causes equality of bond lengths
c) Resonance hybrid always have the lowest energy than any canonical form
Note: The possibility to make a mistake is that you may choose option B. There are ${ 6 }$ equivalent bonds in dichromate ion, not four as all these bonds are in resonance.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Understanding the Electric Field of a Uniformly Charged Ring

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




