




How to Use the Electrochemical Series to Solve Redox and Exam Questions
The electrochemical series is a crucial tool in JEE Main Chemistry, allowing you to rank elements and ions by their standard electrode potentials. Understanding its logic is essential for predicting if a redox reaction will occur spontaneously, balancing redox equations, and solving electrochemistry numericals confidently. The electrochemical series also connects deeply with metallic reactivity and the construction of batteries and voltaic cells, making it indispensable for concept mastery and exam success.
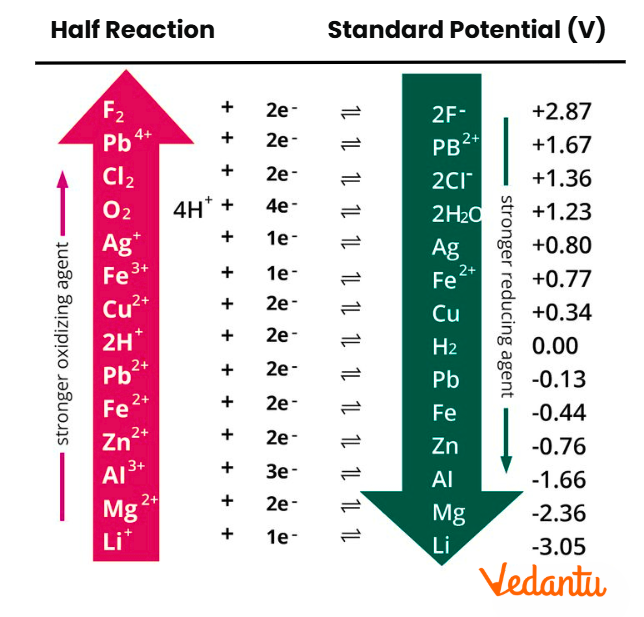
In the context of redox reactions, the electrochemical series arranges substances in order of their tendency to gain or lose electrons, which is reflected in their standard reduction potential (E0) values. Those placed at the top have highly negative potentials and are strong reducing agents; those at the bottom have highly positive potentials and are strong oxidising agents. For JEE Main, this framework helps you decide which half-cell will serve as the anode or cathode, and determines electron flow direction in galvanic cells. Remember, the standard conditions are 298 K, 1 atm, and 1 M concentrations for aqueous ions.
Electrochemical Series: Principle & Arrangement
The guiding principle behind the electrochemical series is the measurement of the tendency of a species to be reduced, relative to the standard hydrogen electrode (SHE), which is arbitrarily set at 0.00 V. Substances are arranged by increasing standard reduction potential:
- Higher (more positive) E0 means a greater tendency to gain electrons (reduced).
- Lower (more negative) E0 means a greater tendency to lose electrons (oxidised).
- Series provides both reactivity order and helps identify strong oxidising/reducing agents.
When solving questions, remember: the substance with a lower E0 will act as the anode (loses electrons), while the higher E0 species acts as the cathode (gains electrons).
Standard Electrode Potentials: Electrochemical Series Table
Below is a simplified, JEE-focused electrochemical series table showing standard reduction potentials (E0, V) at 298 K. More negative values are at the top.
| Half-cell Reaction | E0 (V) | Position in Series |
|---|---|---|
| Li+ + e- → Li(s) | -3.04 | Top (Strongest Reducing Agent) |
| K+ + e- → K(s) | -2.93 | Top |
| Ca2+ + 2e- → Ca(s) | -2.87 | — |
| Na+ + e- → Na(s) | -2.71 | — |
| Mg2+ + 2e- → Mg(s) | -2.37 | — |
| Al3+ + 3e- → Al(s) | -1.66 | — |
| Zn2+ + 2e- → Zn(s) | -0.76 | — |
| Fe2+ + 2e- → Fe(s) | -0.44 | — |
| Ni2+ + 2e- → Ni(s) | -0.25 | — |
| Sn2+ + 2e- → Sn(s) | -0.14 | — |
| Pb2+ + 2e- → Pb(s) | -0.13 | — |
| H+ + e- → ½ H2(g) | 0.00 | Reference (Middle) |
| Cu2+ + 2e- → Cu(s) | +0.34 | Below H2 |
| Ag+ + e- → Ag(s) | +0.80 | Bottom (Strongest Oxidising Agent) |
| Au3+ + 3e- → Au(s) | +1.50 | Bottom |
Standard reduction potential values in this table let you predict reactivity, displacement reactions, and feasibility for cell construction in JEE Main numericals.
The table above can often be found as an "Electrochemical Series Chart" in textbooks and revision materials.
Quick Tricks and Mnemonics for the Electrochemical Series
With so many elements to memorise, smart memory aids are essential. For JEE Main, use mnemonics like:
- Li – Kings Come Not Managing All Zones For New Success Plan. (Order: Li K Ca Na Mg Al Zn Fe Ni Sn Pb...)
- Hydrogen is the middle "dividing line"—metals placed above can displace H2 from acids, while those below generally cannot.
- "Cu Ag Au" are noble metals—lowest reactivity, highest reduction potential.
Link tricky metals with visual cues: "Li at top, noble metals at bottom", "Zn before Fe", "Ag just above Au". Build your own mnemonic to suit your learning style.
Common JEE Applications of the Electrochemical Series
For JEE Main, the electrochemical series helps in three major ways:
- Predicting whether a metal will displace another from a salt solution ("displacement reaction").
- Identifying anode/cathode in a galvanic cell using E0 values, and calculating standard cell EMF with E0cell = E0cathode – E0anode.
- Ranking metals by reactivity for the extraction of elements, and understanding corrosion or electroplating processes.
Key exam tip: Always write half-reactions as reductions when using the table. The element with a higher (more positive) E0 gains electrons (is reduced); the lower one is oxidised.
Solved Example: Predicting the Spontaneity of Redox Reactions
Suppose you are asked: "Will Zn metal displace Cu2+ from solution?"
- Zn2+ + 2e- → Zn(s); E0 = -0.76 V
- Cu2+ + 2e- → Cu(s); E0 = +0.34 V
Calculation:
E0cell = E0cathode – E0anode = 0.34 – (–0.76) = +1.10 V (positive). So, Zn CAN spontaneously reduce Cu2+.
Try similar approaches for all redox-based MCQs, checking the sign and order of E0 values carefully.
JEE Main Preparation: Pitfalls and High-Yield Strategies
Avoid common mistakes like reversing the cathode/anode, copying oxidation potential instead of reduction, or missing standard conditions. For quick review, use concise charts and solve redox & electrochemistry mock test questions.
- Focus MCQ practice on cell EMF, displacement reactions, and order of reducing agents.
- Consult redox reactions and electrochemistry concepts for full topic mastery.
For advanced learners, relate the electrochemical series to topics like inertness of noble metals, corrosion, and smart cell design in practice problems.
Electrochemical Series in JEE: Linked Study Areas
- Review redox reactions, mechanisms, and balancing by oxidation number.
- Master practical chemistry principles and conductivity.
- Use hydrogen reactions and metal extraction as further practice contexts.
- Attempt more questions in the electrochemistry test series.
By building strong intuition for the electrochemical series, you gain confidence for all oxidation-reduction, cell construction, and metal reactivity problems in JEE Main Chemistry. For concise revision, download formula sheets and keep the standard series table handy throughout your preparation. Created and thoroughly reviewed by expert Vedantu Chemistry faculty aligned with the latest JEE syllabus.
FAQs on Electrochemical Series Explained with Table, Tricks, and Applications
1. What is the electrochemical series?
The electrochemical series is a systematic arrangement of elements or ions in order of their standard electrode potentials from most negative to most positive. This series is crucial in predicting redox reaction feasibility, ranking metal reactivity, and understanding electrochemical cell behaviour.
2. How to memorize the electrochemical series?
Students can memorize the electrochemical series effectively by using mnemonics, memory tricks, and regular revision. Some proven strategies include:
- Using a quirky mnemonic phrase for the sequence of elements (e.g., "Please Stop Calling Me A Zebra...")
- Grouping metals and non-metals separately
- Writing the series repeatedly during revision
- Understanding patterns in the order, such as placement of Alkali metals at the top
3. What determines the electrochemical series?
The electrochemical series is determined by the standard electrode potentials (E° values) measured under standard conditions (25°C, 1M, 1 atm). These factors include:
- The inherent tendency of an element or ion to gain or lose electrons (reduction or oxidation)
- Reference to the Standard Hydrogen Electrode (SHE), which is assigned a potential of 0.00 V
- Experimental measurement of half-cell reactions
4. What is the correct electrochemical series?
The correct electrochemical series lists elements/ions such as Li, K, Ca, Na, Mg, Zn, Fe, H, Cu, Ag, Au, etc. according to their standard reduction potential values from negative to positive. You can refer to your textbook's provided table or download an updated PDF for the latest, syllabus-aligned sequence.
5. Why is the electrochemical series important in exams like JEE and NEET?
The electrochemical series helps students quickly determine the feasibility of redox reactions, predict cell potentials, and rank metals for their reactivity—skills tested in JEE, NEET, and CBSE exams. Mastery of the series provides:
- Faster solving of numericals and MCQs
- Better recall during theory questions
- Direct application in competitive exam problem-solving
6. How can I use the electrochemical series to predict the feasibility of a redox reaction?
To predict which redox reaction is feasible, compare the standard electrode potentials (E° values) of the two half-cells:
- The species with a higher (more positive) E° value acts as the oxidizing agent (gets reduced)
- The species with a lower (more negative) E° value acts as the reducing agent (gets oxidized)
- If the overall cell potential (E° cell) is positive, the reaction is feasible (spontaneous)
7. What are some easy tricks or mnemonics to remember the electrochemical series order?
Smart mnemonics and memory tricks help you recall the electrochemical series sequence more easily. For example:
- "Please Stop Calling Me A Crazy Zebra In The Long Haul, Copper Silver Gold" for: K, Na, Ca, Mg, Al, Zn, Fe, Pb, H, Cu, Ag, Au
- Break the list into blocks (active metals, hydrogen, less-reactive metals)
- Associate elements with colours or funny images in your mind
8. Where can I find an easy electrochemical series table for Class 12?
A clear electrochemical series table for Class 12 can be found in NCERT textbooks, exam reference books, or downloadable PDFs on trusted educational websites. Look for tables that list:
- Element or ion
- Their standard reduction potentials (E°)
- Arranged from the most negative to the most positive values
9. What is the difference between the activity series and the electrochemical series?
Activity series ranks metals based on their ability to displace hydrogen (reactivity), while the electrochemical series arranges all elements/ions by their standard electrode potentials (E° values). Key differences:
- Electrochemical series includes non-metals and is based on measured E° values, not just displacement reactions
- Activity series is a simplified version, mainly for metals
10. Can the electrochemical series change based on temperature or pressure?
Yes, the values in the electrochemical series are standard electrode potentials measured at 25°C (298K), 1 atm, and 1M solution. Changing temperature or pressure may alter the potentials, but for exam and numerical purposes, standard values are used unless specifically stated otherwise.
11. How does the electrochemical series relate to corrosion and battery life?
The electrochemical series helps predict corrosion by identifying which metals are easily oxidized. In batteries, it enables selection of suitable anode and cathode materials for maximum cell potential and lifespan.
- Metals higher in the series are more likely to corrode
- Cell voltage depends on the difference in E° values between electrodes
12. Are there exceptions or anomalies in the electrochemical series I should know for competitive exams?
While the electrochemical series is consistent, students should note:
- Some metal values are close, so exam questions may test small order swaps
- Certain complex ions or non-metals behave differently under specific conditions
- Always refer to standard tables provided in your syllabus for up-to-date order
13. Why do some metals appear above hydrogen and others below in the series?
Metals above hydrogen in the electrochemical series have negative electrode potentials and can displace hydrogen from acids (are more reactive). Metals below hydrogen have positive potentials and cannot displace H₂ because they are less reactive.
14. What are the main applications of the electrochemical series?
Key applications of the electrochemical series include:
- Predicting redox reaction spontaneity
- Designing batteries and electrochemical cells
- Ranking metals by reactivity
- Understanding corrosion and its prevention
- Solving JEE, NEET, and CBSE numericals
































