




Overview of Lanthanides and Actinides
Lanthanides and Actinides are radioactive in nature. They consist of 30 elements in total. They contain radioactive elements such as Uranium. These elements are kept in the lanthanide and actinide series of periodic tables. Here, we will learn what are the Lanthanides and Actinides. After reading this article you will be able to answer questions like give the general electronic configuration of lanthanides and actinides, and the number of radioactive elements among lanthanides and actinides. Also, we will learn the applications of lanthanides and actinides.
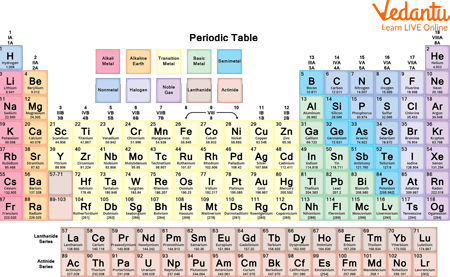
Periodic Table
Lanthanides
Lanthanides contain elements from the atomic numbers 57 to 71. These elements are named after the first element in the group called lanthanum, as it exhibits similar chemical properties to all other elements in the group.
It contains 15 elements and is referred to as Ln by chemists. The elements in this group include Lanthanum, Cerium, Praseodymium, Neodymium, Promethium, Samarium, Europium, Gadolinium, Terbium, Dysprosium, Holmium, Erbium, Thulium, Ytterbium, and lutetium.
These 15 elements are also called Rare Earth Metals and are all silvery-white reactive solid metals obtained from the same ore.
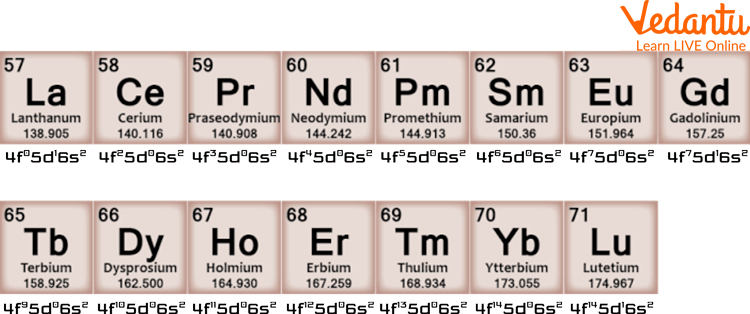
Lanthanides and their Electronic Configuration
Actinides
These are 15 elements that start with the atomic number 89 and go up to the atomic number 103. They are called Actinides as they all display similar chemical properties to the first element of the group Actinium. The symbol An is used to refer to actinides. This group includes mostly man-made elements other than a few that are naturally occurring. It includes Actinium, Thorium, Protactinium, Uranium, Neptunium, Plutonium, Americium, Curium, Berkelium, Californium, Einsteinium, Fermium, Mendelevium, Nobelium, and Lawrencium.
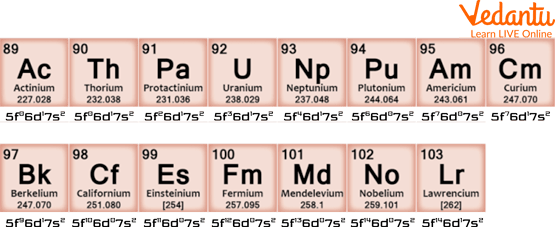
Actinides and their Electronic Configuration
Differences between Lanthanides and Actinides
Differences between Lanthanides and Actinides are listed in the table below.
What Makes These Elements Special?
Actinides are radioactive elements. Due to their property of radioactivity, they are especially useful in nuclear reactions, and they emit radiation. One Lanthanide (Promethium and all its isotopes) is radioactive, while the others are not. The total number of radioactive elements in Lanthanides and Actinides is 16. If someone asks to give the general configuration of Lanthanides and Actinides, you can tell that Actinides have a general configuration of 4f n 5d0 6s2 and that of Lanthanides 5f1-14 6d0-1 7s2
Interesting Facts
Some of the most interesting facts about lanthanides and actinides include:
Lanthanides and Actinides are considered F-block elements as they have one or more electrons in the inner f orbital. However, some are also considered d block elements.
Lanthanides are silver/white coloured metals that tarnish in the air due to their reactivity.
Lanthanides have high melting and boiling points and react with water to produce hydrogen gas.
Many lanthanides burn when exposed to air.
Monazite, Xenotime, and euxenite are the main lanthanide ores.
Uranium and thorium are the most abundant naturally occurring actinides while plutonium is a synthetically obtained actinide.
Actinides have heavy densities except for thorium and americium.
Actinide metals are quite soft.
Lanthanides and Actinides are also called inner transition elements.
The number of radioactive elements among lanthanides and actinides is 16.
Summary
In this article we have discussed all important topics related to lanthanides and actinides. Lanthanides and Actinides are two groups of elements in the periodic table known as inner transition elements. They are mostly metallic elements that are placed below the periodic table in two rows. Actinides are radioactive and consist of elements from atomic numbers 89 to 103. Lanthanides consist of elements with atomic numbers 58 to 71. Combined they consist of 30 elements. Now, you can easily answer questions like give any two differences between lanthanides and actinides. In case of doubts feel free to ask in the comments.
FAQs on What are the Lanthanides and Actinides?
1. What are lanthanides and actinides, and why are they known as inner transition elements?
Lanthanides and actinides are two separate series of metallic elements found at the bottom of the periodic table. They are collectively known as inner transition elements because their differentiating electron enters the (n-2)f orbital, which is an inner orbital compared to the outermost shell. Lanthanides involve the filling of the 4f orbital, while actinides involve the filling of the 5f orbital.
2. What are the key differences between lanthanides and actinides?
The main differences between lanthanides and actinides are:
- Radioactivity: All actinides are radioactive, while among the lanthanides, only Promethium (Pm) is radioactive.
- Oxidation States: Lanthanides predominantly exhibit a stable +3 oxidation state. Actinides show a much wider range of oxidation states (e.g., +3, +4, +5, +6, +7) because the 5f, 6d, and 7s orbitals have comparable energies.
- Complex Formation: Actinides have a greater tendency to form complexes than lanthanides.
- Binding Energy: The 4f electrons in lanthanides have higher binding energy, whereas the 5f electrons in actinides have lower binding energy.
3. Why are lanthanides and actinides placed separately at the bottom of the periodic table?
Lanthanides and actinides are placed separately primarily to maintain the structure and readability of the periodic table. If they were placed within the main body (after Lanthanum and Actinium respectively), the table would become excessively wide and difficult to use. This placement also helps group elements with remarkably similar properties together, highlighting their status as distinct f-block series.
4. What is lanthanoid contraction and what are its major consequences?
Lanthanoid contraction is the steady, gradual decrease in the atomic and ionic radii of lanthanide elements as the atomic number increases. This occurs because the added electrons in the 4f orbital provide a very poor shielding effect, causing the effective nuclear charge to increase and pull the electron shells closer. A key consequence is the remarkable similarity in size between elements of the second and third transition series, for example, Zirconium (Zr) and Hafnium (Hf).
5. Are all lanthanides and actinides radioactive?
No, not all of them are. A major distinction is that all actinide elements are radioactive due to their unstable nuclei. In contrast, for the lanthanides, only one element, Promethium (Pm), is radioactive. All other lanthanides have at least one stable, naturally occurring isotope.
6. Is Lanthanum (La) considered a d-block or an f-block element?
This is a point of common confusion. Technically, Lanthanum (La) is a d-block element because its differentiating electron enters the 5d orbital (electronic configuration: [Xe] 5d¹6s²). However, its chemical properties are extremely similar to the f-block elements that follow it. For this reason, it is almost always studied along with the lanthanide series for chemical and practical convenience.
7. What are some important real-world examples of how lanthanides and actinides are used?
Both series have significant applications:
- Lanthanides: They are widely used to create strong metal alloys. For instance, mischmetal, an alloy of lanthanide metals, is used in cigarette lighters. They also serve as catalysts in the petroleum industry for refining crude oil and are crucial components in optical devices like night-vision goggles and lasers.
- Actinides: Their radioactivity is their most useful property. Uranium (U) and Plutonium (Pu) are primary fuels in nuclear power plants and nuclear weapons. Americium (Am) is used in household smoke detectors.
8. How do the oxidation states of actinides compare with those of lanthanides?
Actinides exhibit a much wider variety of oxidation states compared to lanthanides. Lanthanides show a dominant and very stable +3 oxidation state, with +2 and +4 states being relatively rare. In contrast, actinides can show oxidation states from +3 up to +7. This is because the energies of the 5f, 6d, and 7s orbitals are very close, allowing electrons from all three subshells to participate in chemical bonding.









