




How Karl Fischer Titration Works and Why It Matters in Chemistry
Karl Fischer titration is a titration method that uses volumetric or coulometric titration to determine the quantity of water present in a given analyte. This method for quantitative chemical analysis was developed by the German chemist Karl Fischer in 1935. Karl Fischer reagent consists of iodine, sulphur dioxide, a base and a solvent, such as alcohol. In the aqueous environment, the Bunsen reaction between iodine and sulphur dioxide is the basis for the reactions of Karl Fischer reagents.
Karl Fischer Titration Principle
The principle of Karl Fischer titration is predicated on the oxidation reaction between iodine and sulphur dioxide. Water reacts with iodine and sulphur dioxide to form sulphur trioxide and hydrogen iodide. This reaction is named the Bunsen reaction. When all the water is consumed that’s the endpoint.
Karl Fischer Titration Formula
Karl Fischer titration is a technique for the determination of moisture content. This method is based on a reagent which reacts with water and converts the water into a non-conductive chemical. Water determination:
\[{\rm{water}}\,{\rm{content}} = \dfrac{{SF \times 100}}{{{\rm{mg}}\,{\rm{of}}\,{\rm{test}}\,{\rm{preparation}}}}\]
where S is the volume in mL of the reagent consumed and F is the water per mL of the reagent.
Karl Fischer Titration Instrumentation
Karl Fischer titration is based on iodide reaction, which means water reacts with iodine. The apparatus consists of an automatic burette, a back titration flask, a stirrer, and equipment for amperometric titration at a constant voltage or potentiometric titration at a constant current.
Coulometric KF analysis requires only one, iodide-containing solution. Iodine needed for KF reaction is produced by anodic oxidation of iodide from solution and the end point is detected electrochemically. Coulometric determination is best suited for samples with less than 1% of water.
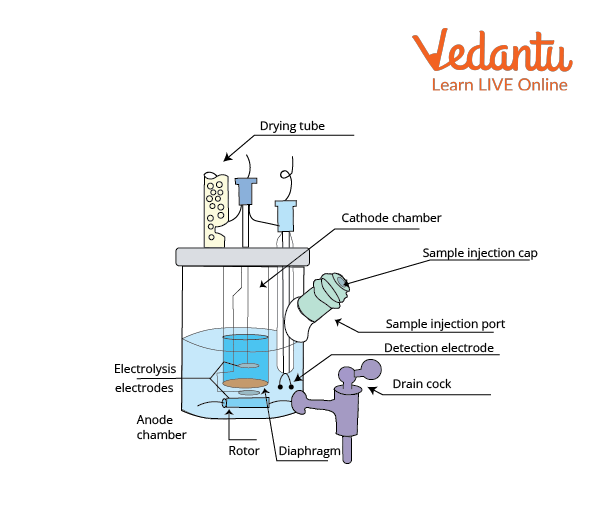
Electrolysis Cell of Coulometric Moisture Metre
Volumetric determination is suitable for the determination of water content of less than 1% of water. The sample is dissolved in KF solvent (usually methanol based) and therefore, the iodine is added as a part of the KF reagent containing sulphur dioxide and iodine dissolved in pyridine and methanol. The endpoint is determined potentiometrically.
Karl Fischer Titration Reaction
The alcohol reacts with sulphur dioxide and base to form an intermediate alkyl sulfite salt, which is then oxidised by iodine to an alkyl sulphate salt. This oxidation reaction consumes water.
Step 1:
\[C{H_3}OH + S{O_2} + RN \to \left[ {RNH} \right]S{O_3}C{H_3}\]
Step 2:
\[{H_2}O + {I_2} + {\left[ {RNH} \right]^ + }S{O_3}CH_3^ - + 2RN \to {\left[ {RNH} \right]^ + }S{O_4}CH_3^ - + 2{\left[ {RNH} \right]^ + }{I^ - }\]
Important Questions and Answers
Karl Fischer is a widely used method for the determination of water in the pharmaceutical industry. Karl Fischer is employed to determine the only water content present in the sample but no other volatile impurities in the samples. In interviews on quality control of pharmaceutical industries, it's generally asked. Karl fischer titration interview questions and answers are listed below:
1. What are the two methods of water determination?
Ans:
Volumetric Water Determination
Coulometric Water Determination
2. What is the difference between volumetric Karl Fischer determination and coulometric Karl Fischer determination?
Ans: The titrant is added directly through the burette in the volumetric determination while titrant is generated electrochemically within the titration vessel in coulometric titration.
3. What is the basis of Karl Fischer titration?
Ans: The basis of Karl Fischer titration is predicated on the oxidation reaction between iodine and sulphur dioxide. Water reacts with iodine and sulphur dioxide to produce sulphur trioxide and hydrogen iodide. An endpoint is reached when all the water is consumed.
Key Features of Karl Fischer Titration
The volumetric Karl Fischer titration method is employed to determine water content by adding iodine-containing titrant to the sample which is then dissolved or dispersed in a suitable solvent.
Karl Fischer titration is a redox reaction which uses the consumption of water during the reaction to measure the amount of water in a sample.
It is the reference method for water determination because of its specificity, accuracy, and speed of instrument.
The optimum pH range of the sample solution is 5.5 to 8.
FAQs on Karl Fischer Titration: Definition, Method & Applications
1. What is the fundamental principle of Karl Fischer titration?
The Karl Fischer titration is based on a redox reaction first studied by Robert Bunsen. The core principle is that iodine (I₂) reacts stoichiometrically with water (H₂O) in the presence of sulfur dioxide (SO₂), a base (like imidazole), and a suitable solvent (typically methanol). The amount of iodine consumed is directly proportional to the amount of water present in the sample, allowing for precise water content determination.
2. What are the key chemical reactions involved in Karl Fischer titration?
The process involves two main steps. First, the alcohol (ROH, usually methanol CH₃OH) reacts with sulfur dioxide and a base (B) to form an alkyl sulfite intermediate. In the second step, this intermediate is oxidized by iodine in the presence of water.
Step 1: SO₂ + CH₃OH + B → [BH]⁺SO₃CH₃⁻
Step 2: [BH]⁺SO₃CH₃⁻ + I₂ + H₂O + 2B → [BH]⁺SO₄CH₃⁻ + 2[BH]⁺I⁻
Essentially, for every one mole of water, one mole of iodine is consumed.
3. Why is methanol specifically used as a solvent in KF titration?
Methanol serves two critical functions in Karl Fischer titration. Firstly, it acts as a solvent, dissolving the sample and the reagents (iodine and sulfur dioxide). Secondly, it is a reactant itself, participating in the formation of the methyl sulfite intermediate, which is essential for the reaction with water to proceed at an appropriate rate. Its use ensures the reaction occurs in a non-aqueous, controlled environment.
4. What is the difference between volumetric and coulometric Karl Fischer titration?
The primary difference lies in how the iodine is introduced and measured to react with water. This choice depends on the expected water content in the sample.
- Volumetric Titration: A solution with a known concentration of iodine (the KF reagent) is added to the sample using a burette. The volume of the reagent used determines the water content. It is best suited for samples with higher water content, typically from 100 ppm to 100%.
- Coulometric Titration: Iodine is generated electrochemically in the titration cell from an iodide-containing solution. The amount of charge (coulombs) needed to generate enough iodine to react with all the water is measured. This method is extremely sensitive and ideal for samples with very low water content, from 1 ppm to 5%.
5. How is the endpoint detected in a Karl Fischer titration?
The endpoint in modern Karl Fischer titrators is detected using an electrochemical method called biamperometric indication. A constant current is applied across a double platinum electrode submerged in the solution. Before the endpoint, there is no free iodine, and the solution exhibits high resistance. Once all the water has been consumed, even a tiny excess of iodine causes a sudden drop in resistance and a sharp increase in current, which signals the endpoint of the titration.
6. What are some common sources of error in Karl Fischer titration and how can they be avoided?
Accurate KF titration requires careful technique to avoid errors. Common sources include:
- Atmospheric Moisture: Ambient humidity can be absorbed by the solvent, leading to falsely high readings. This is avoided by using a sealed titration vessel with drying tubes.
- Side Reactions: Aldehydes and ketones can react with the methanol in the reagent to form acetals and ketals, releasing water and causing inaccurate results. Using specialized aldehyde & ketone-suppressing KF reagents can prevent this.
- Incorrect Sample Handling: Improperly stored samples or inaccurate weighing can introduce significant errors. Samples should be kept in sealed containers, and an analytical balance must be used for precise measurement.
7. Why is Karl Fischer titration considered a superior method for water determination compared to loss-on-drying?
Karl Fischer titration is considered the gold standard for water determination due to its high specificity, accuracy, and speed. Unlike the loss-on-drying method, which measures the loss of any volatile substance upon heating, KF titration is a chemical method that reacts specifically with water. This means it can distinguish water from other volatile components like alcohols or solvents, leading to a much more accurate and true measurement of water content, which is critical for quality control in industries like pharmaceuticals, food, and petrochemicals.
























