




Key Physical & Chemical Properties of Diethyl Ether
The organic compound diethyl ether is made up of two carbon atoms connected by an oxygen atom (C-O-C). It is a colourless, highly flammable, highly volatile liquid with an ethereal odour (sweet smelling). The molecular formula of diethyl ether is $C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}$ and the IUPAC name of diethyl ether is ethoxyethane.
Valerius Cordus synthesised diethyl ether in the year 1540 and gave it the name "sweet oil of vitriol" (oleum dulce vitrioli), reflecting the fact that it is produced by distilling a mixture of ethanol and sulfuric acid. August Sigmund Frobenius named the substance "ether" in 1729. It is frequently used as an engine starter fluid and as a solvent in laboratories. Before non-flammable medications like halothane were created, it was used as a general anaesthetic. Intoxicating recreational drugs have been made from it in the past.
Structure of Diethyl Ether
Diethyl ether usually referred to as ethyl ether, is an ether-class chemical molecule with the formula ${{({{C}_{2}}{{H}_{5}})}_{2}}O$. R-O-R. It is used to signify diethyl ether. Here, R stands for the alkyl group(${{C}_{2}}{{H}_{5}}$), and O stands for the oxygen atom.
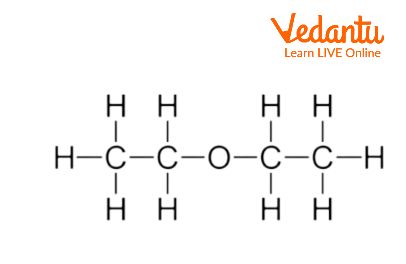
Structure of Diethyl Ether
Physical Properties of Diethyl Ether
Diethyl ether is a colourless liquid with a molar mass of 74.123 g/mol.
It is highly volatile.
It is highly flammable.
It has a sweetish pungent odour.
The boiling point is 34.6 °C (94.3 °F; 307.8 K).
The melting point is -116.3 °C (-177.3 °F, 156.8 K).
Its density is 0.7134 $\mathrm{g}/\mathrm{cm}^3$ , liquid.
Its vapour pressure is 440 mmHg at 20 °C (58.66 kPa at 20 °C).
Chemical Properties of Diethyl Ether
Combustion: Due to its high flammability, diethyl ether undergoes a combustion reaction to form carbon dioxide and water as products.
${{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\,+\,6{{O}_{2}}\,\to \,4C{{O}_{2\,}}+\,5{{H}_{2}}O$
Halogenation: On halogenation with halogens like chlorine or bromine, diethyl ether undergoes a substitution reaction to form halo-substituted ether in the absence of sunlight.
${{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\,+\,C{{l}_{2}}\,\to \,{{C}_{2}}{{H}_{4}}(Cl)O{{C}_{2}}{{H}_{4}}(Cl)$
Preparation of Diethyl Ether
Diethyl ether is produced by heating dry silver oxide with ethyl iodide.
Williamson synthesis: This process involves heating sodium or potassium ethoxide with chloro, bromo, or iodo ethane. Diethyl ether and sodium iodide are the products of this reaction.
${{C}_{2}}{{H}_{5}}ONa\,+\,{{C}_{2}}{{H}_{5}}I\,\to \,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\,+\,NaI$
Diethyl ether is produced by heating ethyl alcohol with alumina (Al2O3) at 250°C.
${{C}_{2}}{{H}_{5}}OH\,\xrightarrow[250]{A{{l}_{2}}{{O}_{3}}}\,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\,+\,{{H}_{2}}O$
Laboratory method: A high concentration of ethyl alcohol is combined with a less concentrated sulfuric acid, and the resultant mixture is heated to roughly 140°C to produce diethyl ether.
${{C}_{2}}{{H}_{5}}OH\,+\,{{H}_{2}}S{{O}_{4}}\,+\,{{C}_{2}}{{H}_{5}}HS{{O}_{4}}\,+\,{{H}_{2}}O$
${{C}_{2}}{{H}_{5}}HS{{O}_{4}}\,+\,{{C}_{2}}{{H}_{5}}OH\,\to \,{{C}_{2}}{{H}_{5}}O{{C}_{2}}{{H}_{5}}\,+\,{{H}_{2}}S{{O}_{4}}$
Health Hazards of Diethyl Ether
Highly flammable liquid and vapour and it may also form explosive peroxides.
It is very harmful if swallowed and causes drowsiness, dizziness, and may induce vomiting.
Continual exposure to ether could result in skin dryness or cracking and may also damage the proper functioning of kidneys.
Uses of Diethyl Ether
It is used as a fuel:
Due to its high volatility and low flash point, diethyl ether, which has a high cetane number of 85–96, is utilised as a starting fluid in combination with petroleum distillates for gasoline and diesel engines.
Chemistry:
A typical aprotic solvent used in laboratories is diethyl ether.
It dissolves in water at a rate of 1.5 g/100 g (1.0 g/100 ml) and has low solubility in water (6.05 g/100 ml at 25 °C). This makes it perfect for use as a non-polar solvent in liquid-liquid extraction, along with its high volatility.
In addition to other reactions employing organometallic reagents, the Grignard reaction uses it as a typical solvent.Anesthesia:
Due to ether's better therapeutic index, or a bigger gap between an effective dose and a potentially toxic dose, diethyl ether generally replaced the use of chloroform as a general anesthetic.
Recreational use:
Ether is a recreational substance because of its anesthetic and euphoric properties. A long history of recreational use exists for the inhalant diethyl ether in anesthetic dosage.
Important Questions
1. Name a few physical properties of diethyl ether.
Ans: Diethyl ether is an organic compound that is colorless, ethereal odoured, highly flammable, and highly volatile liquid with a melting point and boiling point of -116.3 °C and 34.6 °C respectively.
2. Mention a method to prepare diethyl ether.
Ans: Diethyl ether can be prepared by distilling highly concentrated ethyl alcohol with sulphuric acid at about 140 degrees Celsius.
Summary
Ethyl ether, commonly referred to as diethyl ether, is a popular anesthetic that is a member of the large class of chemical compounds known as ethers. The molecular formula and the IUPAC name of diethyl ether are $C{{H}_{3}}C{{H}_{2}}OC{{H}_{2}}C{{H}_{3}}$ and ethoxyethane, respectively. It is a colourless, ethereal odoured, highly flammable, and highly volatile liquid with a melting point and boiling point of -116.3 °C and 34.6 °C, respectively. It is commonly used as a laboratory solvent, fuel, and anesthetic.
Practice Questions
1. Which one of these is not a synonym of diethyl ether?
Ethyl ether
Ethoxyethane
Ethoxymethane
Ether
2. Which of these is a structural isomer of diethyl ether?
Butanal
Butanol
Butane
Butene
Answers
(c)
(b)
FAQs on Diethyl Ether: Complete Chemistry Guide
1. What is diethyl ether, and what is its IUPAC name?
Diethyl ether is a simple ether, an organic compound with the chemical formula (C₂H₅)₂O. It consists of two ethyl groups attached to a central oxygen atom. According to the IUPAC nomenclature system, its official name is ethoxyethane. It is a colourless, highly volatile, and flammable liquid with a characteristic sweetish odour.
2. What are the main physical properties of diethyl ether?
The key physical properties of diethyl ether, relevant as per the CBSE syllabus, include:
- State: It is a colourless, volatile liquid at room temperature.
- Odour: It has a characteristic sweet, pungent smell.
- Boiling Point: It has a very low boiling point of 34.6 °C (307.7 K).
- Solubility: It is only sparingly soluble in water but is miscible with most organic solvents like alcohol, benzene, and chloroform.
- Density: It is less dense than water.
3. How is diethyl ether commercially prepared using Williamson synthesis?
Diethyl ether is prepared via the Williamson synthesis by reacting sodium ethoxide with ethyl chloride (or another ethyl halide). The ethoxide ion (C₂H₅O⁻) acts as a nucleophile and attacks the ethyl chloride molecule, displacing the chloride ion in an Sₙ2 reaction to form ethoxyethane (diethyl ether) and sodium chloride as a byproduct. The reaction is: C₂H₅O⁻Na⁺ + C₂H₅Cl → C₂H₅OC₂H₅ + NaCl.
4. Why is diethyl ether a commonly used solvent in organic chemistry, especially for Grignard reactions?
Diethyl ether is an excellent solvent for many organic reactions due to its two primary characteristics. Firstly, it is largely unreactive (aprotic) and does not interfere with most reagents. Secondly, the lone pairs of electrons on its oxygen atom can effectively solvate and stabilise cations, such as the magnesium ion in a Grignard reagent (R-Mg-X). This stabilisation prevents the reagent from decomposing and maintains its reactivity.
5. What are the dangers associated with storing diethyl ether, and how are they mitigated?
The primary danger of storing diethyl ether is its tendency to react slowly with atmospheric oxygen in the presence of light to form highly explosive peroxides. These peroxides are less volatile than the ether itself and can concentrate during distillation, potentially leading to a violent explosion. To mitigate this, ether is stored in dark, airtight containers, and its purity is often tested for peroxides before use, which can be removed by washing with a solution of ferrous sulfate (FeSO₄).
6. Why does diethyl ether have a much lower boiling point (34.6 °C) than its isomer, butan-1-ol (117.7 °C)?
This significant difference in boiling points is due to the types of intermolecular forces present. Butan-1-ol has a hydroxyl (-OH) group, which allows its molecules to form strong hydrogen bonds with each other. Diethyl ether lacks this -OH group and can only form weaker dipole-dipole interactions and van der Waals forces. Since much more energy is required to overcome the strong hydrogen bonds in butan-1-ol, its boiling point is substantially higher.
7. What is the outcome of the reaction when diethyl ether is treated with excess hot concentrated HI?
When diethyl ether (C₂H₅OC₂H₅) is heated with an excess of concentrated hydroiodic acid (HI), the ether linkage is cleaved. The reaction proceeds in two steps. First, one molecule of iodoethane (ethyl iodide) and one molecule of ethanol are formed. Since HI is in excess and the reaction is heated, the ethanol formed is further converted into another molecule of iodoethane. The overall reaction is: C₂H₅OC₂H₅ + 2HI (hot, excess) → 2C₂H₅I + H₂O.
8. Is diethyl ether still used as a general anaesthetic?
While historically significant as one of the first general anaesthetics, the use of diethyl ether has largely been discontinued in developed countries. This is due to several disadvantages, including its high flammability, slow onset of anaesthesia, and unpleasant post-operative effects like nausea. However, it is still used in some developing nations because of its low cost and high therapeutic index, which means there is a wide margin between the effective dose and a toxic dose.
























