




How Does a Bomb Calorimeter Measure Heat in Reactions?
To test the calorific value of liquid and solid fuels that are traded based on the value, we tend to use a bomb calorimetry. Fuels like coal and oil must meet rules specifying the overall calorific value to make sure the standard and purity of the fuel. The heat that's measured is stated as the change of internal energy (E). The heat changes of a reaction are often monitored in chemistry at a fixed pressure or volume. How to use a bomb calorimeter, construction of bomb calorimeter, how does a colorimeter work, bomb calorimetery calculations and many more concepts will be discussed in this article.
What is Bomb Calorimeter?
The calorimeter used to verify the energy change throughout a reaction is known as a bomb calorimeter. The calorimeter of Berthelot is the origin of the modern bomb calorimeter.
Bomb calorimeters should resist the high pressure inside the calorimeter, whereas measuring the response. The fuel is ignited using electrical energy; once the fuel burns, it heats up the surrounding air that expands and exits through a tube leading out of the calorimeter. Once air escapes through the copper tube, it heats the water outside the tube also. The calorie content of the fuel is also calculated using the temperature change within the water.
To test the calorific value of solids and liquid fuels bomb calorimeters are used. If the heat capability of the calorimeter is known, we are able to determine the change in heat by noting the temperature. Fuel like coal and oil must meet rules specifying the entire calorific value.
Construction of Bomb Calorimeter
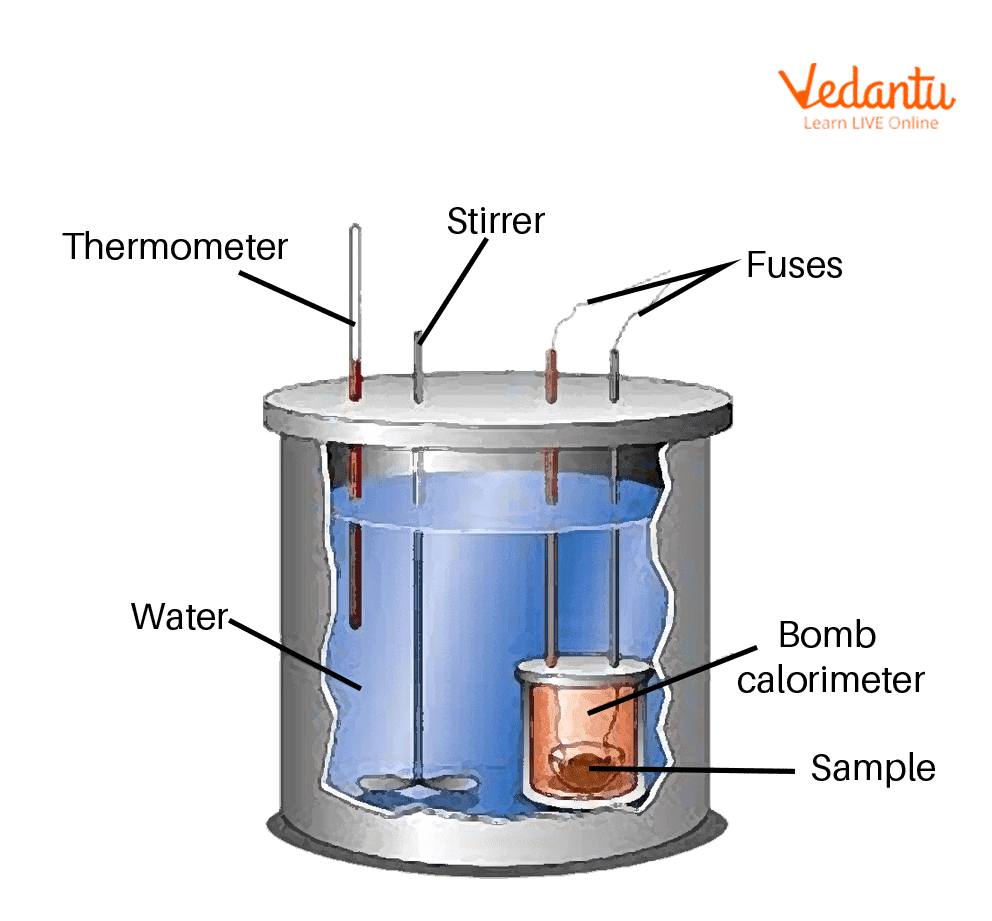
Bomb Calorimeter
Bomb calorimetery calculations are performed as follows:
The sample, oxygen, the stainless-steel bomb, and water compose the bomb calorimeter's main parts.
The wall stops heat from escaping from the calorimeter into the rest of the cosmos, i.e.
q calorimeter = 0.
The combustion process happens at a fixed volume and there's no work because the bomb is constructed of stainless-steel.
Wc calorimeter = - p dV = 0
Thus, the change in internal energy, dU, for the calorimeter is zero.
dU calorimeter = q calorimeter + w calorimeter = 0
The thermodynamic interpretation of this equation is that the calorimeter is isolated from the remainder of the universe.
Working of Bomb Calorimeter
The bomb is used to determine the hot values of liquid and solid fuels. It consists of a robust steel shell referred to as a bomb. The calorimeter consists of steel that has excellent corrosion resistance and may sustain high pressures. Within the calorimeter, a robust cylindrical bomb is used to cause combustion. Just at the apex of the bomb, there ar 2 values. One provides O to a bomb, whereas the other emits fumes.
The bomb is placed within a copper vessel that contains water. Inside the calorimeter, there's a stirring device for agitating the water. The calorimeter containing the bomb is placed in another container that acts as a heat insulator. Using the thermometer the temperature of water in the calorimeter is measured.
The calorimeter is additionally equipped with a water and air jacket to decrease light losses. Through the lid of the calorimeter, a stirrer keeps the temperature of the water consistent, and a thermometer with a temp precision of 0.001 degree C is installed. The surroundings are completely different and also the calorimeter absorbs the heat generated by the fuel during burning.
Uses of Bomb Calorimeter
Bomb calorimeter set up is used primarily in the scientific study of thermodynamical processes. It measures the heat of combustion produced in a chemical process. Also, it measures changes in physical property throughout the reaction.
They are used to test the hot value of liquid and solid fuels that are traded based on the calorific values. Fuels like coal and oil should meet the rules regarding the overall calorific value to make sure the standard and purity of the fuel. Bomb calorimetry may test liquid fuels like gasolene and lamp oil.
It may determine if the industries are using unsafe waste as an alternate fuel by generating the values.
It can also make sure the calorie content of a product, so we are able to examine the content in food on humans, and also thereby extend into nutritionary concerns and the effect of diet on the body.
We can also find the heat of detonation of test propellants and explosives.
Key Features
A bomb calorimeter consists of small sample cup, oxygen, a stainless-steel bomb, water, a stirrer, a thermometer, a dewar or an insulating bottle and an ignition circuit.
The reaction takes place only in water after we talk about bomb calorimeter.
It can measure heat flow of the reaction, which can be equal to magnitude of enthalpy change.
When measuring heat prospective or the basic heat of a substance, many students use calorimeters. A calorimeter is employed to calculate the overall heat energy and subsequently to measure the precise heat of a substance or other heat related data.
FAQs on Bomb Calorimeter: Principle, Construction & Uses
1. What is a bomb calorimeter primarily used to measure?
A bomb calorimeter is a specialised instrument used to determine the heat of combustion of solid and liquid fuels or food samples. The experiment is conducted at a constant volume, which means the value it directly measures is the change in internal energy (ΔU) for the reaction.
2. What are the essential components of a bomb calorimeter and their functions?
A bomb calorimeter consists of several key parts that work together to ensure accurate measurements:
- Steel Bomb: A strong, sealed container made of steel where the sample is placed and ignited under high pressure of pure oxygen to ensure complete combustion.
- Calorimeter Vessel: An insulated container filled with a known mass of water that surrounds the bomb. It absorbs the heat released by the combustion.
- Stirrer: A mechanical stirrer that continuously circulates the water in the vessel to ensure a uniform temperature distribution.
- Thermometer: A high-precision thermometer (like a Beckmann thermometer) to accurately measure the change in water temperature before and after the reaction.
- Ignition Wires: Wires that pass an electric current to a fuse wire inside the bomb, initiating the combustion of the sample.
3. How is the heat of a reaction calculated using data from a bomb calorimeter?
The heat evolved during the combustion (q) is absorbed by the water and the calorimeter itself. It is calculated using the formula: q = Ccal × ΔT, where Ccal is the heat capacity of the entire calorimeter system (including water) and ΔT (Tfinal - Tinitial) is the measured change in temperature. Since this heat is measured at constant volume, it is equal to the change in internal energy for the reaction (q = ΔU).
4. Why does a bomb calorimeter measure the change in internal energy (ΔU) and not the change in enthalpy (ΔH)?
A bomb calorimeter measures the change in internal energy (ΔU) because the reaction occurs in a sealed, rigid steel container (the 'bomb'). This means the volume remains constant throughout the process (ΔV = 0). According to the first law of thermodynamics, work done (w) is -PΔV. Since ΔV is zero, no work is done (w = 0). Therefore, the change in internal energy (ΔU = q + w) becomes equal to the heat exchanged at constant volume (ΔU = qv). In contrast, enthalpy (ΔH) is defined as the heat exchanged at constant pressure (ΔH = qp).
5. What is the real-world importance of determining the calorific value of coal using a bomb calorimeter?
Determining the calorific value of coal is crucial for industrial and commercial purposes. The heat of combustion value obtained from a bomb calorimeter indicates the energy content and quality of the coal. This information helps power plants determine the efficiency of coal as a fuel, allows industries to calculate the amount of fuel needed for specific processes, and sets the commercial price of the coal based on its energy output.
6. How does a bomb calorimeter differ from a simple coffee-cup calorimeter?
The primary difference lies in the conditions under which they operate:
- Operating Condition: A bomb calorimeter operates at constant volume, measuring the change in internal energy (ΔU). A coffee-cup calorimeter operates at constant pressure (atmospheric pressure), measuring the change in enthalpy (ΔH).
- System Type: The bomb calorimeter is a sealed, rigid system designed for high-pressure combustion reactions. A coffee-cup calorimeter is a simpler, often unsealed system used for reactions in aqueous solutions.
- Applications: A bomb calorimeter is used for combustion reactions (e.g., finding the calorific value of fuels), while a coffee-cup calorimeter is used for processes like dissolution, neutralisation, or simple solution reactions.
7. What are the common sources of error in a bomb calorimeter experiment?
To ensure accuracy, several potential errors must be minimised:
- Incomplete Combustion: If the oxygen supply is insufficient or the sample is not finely powdered, it may not burn completely, leading to a lower heat reading.
- Heat Loss: Heat can be lost from the calorimeter to the surroundings. This is minimised by using an insulated jacket and applying a cooling correction.
- Temperature Measurement: Inaccurate readings from the thermometer can significantly affect the final calculation. A high-precision, calibrated thermometer is essential.
- Side Reactions: Formation of byproducts like nitric acid (from nitrogen in the air) can contribute to the heat, requiring a correction in the final calculation.
























