




An Overview of Class 12 Chemistry To Set Up Simple Daniell Cell Using Salt Bridge And Determine Its Emf Experiment
Introduction
Daniell Cells are used to either generate or store electricity. Daniell Cells are used in the development of batteries and electrical telegraphy. Battery means collections of cells.
Table of Contents
Aim of the Experiment
Apparatus Required
Theory
Prodedure
Observations
Result
Precautions
Aim of the Experiment
To set up simple Daniell cell using salt bridge and determine its emf
Apparatus Required
One beaker
a porous pot
connecting wires
milli voltmeter
sand paper
zinc strip
copper strip
1 M ZincSulphate solution
1 M CopperSulphate solution
Theory
When a copper electrode dipped in copper sulphate solution is connected to a zinc electrode dipped in zinc sulphate solution, electrons flow from zinc electrode to copper electrode and the following chemical reactions occur:
$${{Zn}}$$ $$\to$$ $${{Zn}^{2+}{+}{2}{e}^{-}}$$
$${{Cu}^{2+}{+}{2}{e}^{-}}$$ $$\to$$ $${{Cu}}$$
The overall reactions is $${{Zn}{+}{Cu}^{2+}}$$ $$\to$$ $${{Zn}^{2+}{+}{Cu}}$$
While doing the experiment you should assume that the beaker with a name. For example Beaker “A”. So as to avoid confusion.
Procedure
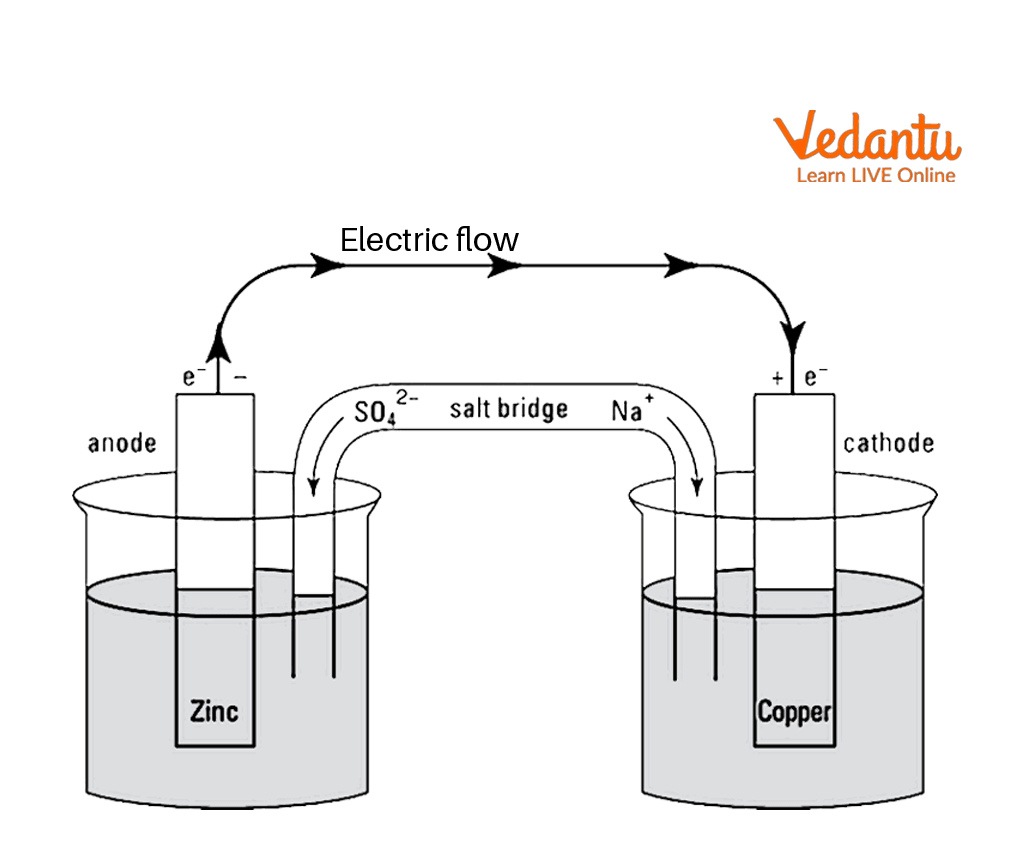
Daniell Cell with salt bridge
Set up the apparatus as shown in the above figure.
The flow of electrons will happen for sometime. Now note the readings of voltmeter.
Observations
Result
The EMF varies non-linearly as the concentration of reactants changes.
Precautions
Copper sulphate and zinc sulphate concentrations should be neither too low nor too high.
The porous pot should not be completely immersed in the copper sulphate solution, and no copper sulphate solution should enter the porous pot.
Sand the zinc and copper strips before using them.
Dilute the solution slowly and carefully.
Only take note of the reading when the pointer becomes stable.
Connect the copper strip to the voltmeter's positive terminal and the zinc strip to the negative terminal.
Lab Manual Questions
1. In this experiment, explain how le Chatelier's principle applies to your electrochemical cell.
Ans. Le Chatelier's principle states that if a dynamic equilibrium is disturbed by changing the conditions, the position of equilibrium shifts to counteract the change to reestablish equilibrium.
2. Is the Nernst law followed across the entire concentration range?
Ans. The value of the equilibrium potential for any ion depends upon the concentration gradient for that ion across the membrane. If the concentrations on the two sides were equal, the force of the concentration gradient would be zero, and the equilibrium potential would also be zero.
3. Is Daniel cell a type of primary cell?
Ans. Daniel cell is a primary cell which produces an electromotive force between its two terminals by a series of chemical reactions. It is composed of copper and zinc with the cuprite sulphate as an electrolyte.
4. In Daniell's cell, which solution is used?
Ans. In a Daniell cell, the electrolytes are $${{Zn}{S}{O}_{4}}$$(aq) with a Zn anode in its half-cell and $${{Cu}{S}{O}_{4}}$$(aq) with a copper cathode in its half-cell.
Viva Questions
1. What are the two electrodes that are used in the Daniell cell?
Ans. Zinc and Copper Electrodes are used.
2. How does a Daniel cell function?
Ans. In a Daniell cell, electrons flow from zinc electrode to copper electrode through an external circuit, while metal ions form one half cell to the other.
3. How do you depict Daniell cell?
Ans. The symbol double vertical lines || represents the Daniell cell. The left part is oxidation half Cell and the right part is reduction half Cell.
4. Why can't Daniell cell be recharged?
Ans. Daniell Cell is not rechargeable, because recharge would much aggravate the Cu2+ crossover, indicating a battery-killing process.
5. Is Daniel cell a dry cell?
Ans. No. Daniell cell is a wet cell.
6. What is the Daniell cell Nernst equation?
Ans. $${{E}^{0}_{cell}{=}{\frac{0.0592}{n}}{log}{K}}$$
7. Is the galvanic cell and the Daniel cell the same thing?
Ans. A galvanic cell is one which converts the redox reaction chemical energy in to electrical energy through outside circuit. But a Daniel cell is the cell constructure by redox couple of Zn|$${{Zn}{S}{O}_{4}}$$ and Cu|$${{Cu}{S}{O}_{4}}$$. So Daniel cell is primarily a Galvanic cell but all the galvanic cells are not Daniel cell.
8. Which phenomena does not occur in Daniell cell?
Ans. There is no change in colour of the $${{Zn}{S}{O}_{4}}$$ solution.
9. Is a Daniel cell a spontaneous reaction?
Ans. The emf of the cell is +1.1V. hence the cell reaction is spontaneous.
10. What is Daniell's cell used for?
Ans. The Daniell cell can be used to 'generate' electricity by consuming an electrode or to store electricity. Although the Daniell cell was one of the early examples of a device for generating electricity, it is relatively difficult to analyse thermodynamically because it has electrodes of different materials.
Practical-Based Questions
1. An electrochemical cell is typically made up of a cathode and an anode. Which of the following statements about the cathode is correct?
a) Oxidation occurs at the cathode
b) Electrons move into the cathode
c) Usually denoted by a negative sign
d) Usually made up of insulating material
Answer: B
2. Cell emf is the sum of the electrode potentials of the two electrodes when no current is drawn through an electrochemical cell. True or False?
a) True
b) False
Answer: A
3. An electrochemical cell can convert electrical energy to chemical energy.
a) True
b) False
Answer: B
4. When equilibrium is reached inside the two half-cells of the electrochemical cells, what is the net voltage across the electrodes?
a) > 1
b) < 1
c) = 0
d) Not defined
Answer: C
5. Which of the following statements is correct regarding Electrochemical cells?
a) Cell potential is an extensive property
b) Cell potential is an intensive property
c) The Gibbs free energy of an electrochemical cell is the intensive property
d) Gibbs free energy is undefined for the electrochemical cell
Answer: B
6. Which of the following factors does not affect the electrode potential of an electrode?
a) Nature of the electrode (metal)
b) Temperature of the solution
c) Molarity of the solution
d) Size of the electrode
Answer: D
7. Why are the saturated solutions of electrolytes for the salt bridge prepared in agar-agar jelly or gelatin?
a) The jelly acts as an electrolyte
b) It help the electrolytes to mix with the contents of the half cells
c) It help maintain the electrical polarity between the two half-cell solutions
d) It keep the electrolyte in semi-solid phase and prevents it from mixing with the two half-cell solutions
Asnwer: D
8. Which of the following is not a type of electrochemical cell?
a) Voltaic cell
b) Photovoltaic cell
c) Electrolytic cell
d) Fuel Cell
Answer: B
9. EMF of Daniell cell is
a)1.1 volts
b)2.1 volts
c)1.0 volts
d)1.5 volts
Answer: A
10. What is the observation when the opposing external applied potential to an electrochemical cell is greater than the cell’s potential?
a) The electrochemical cell behaves like an electrolytic cell
b) The electrochemical cell stops functioning
c) Only oxidation reactions occur in the cells
d) Only reduction reactions occur in the cells
Answer: A
Conclusion
A Daniell Cell is an ElectroChemical Cell that performs chemical reactions to generate electricity. The anode in the Daniell Cell is zinc metal, and the cathode is copper.
Daniell Cells are used to either generate or store electricity. Daniell Cells are used in the development of batteries and electrical telegraphy. Battery means collections of cells.
FAQs on Class 12 Chemistry To Set Up Simple Daniell Cell Using Salt Bridge And Determine Its Emf Experiment
1. What is the primary function of a salt bridge in a Daniell cell, and what are the consequences for the cell's operation if it is removed?
The primary function of a salt bridge in a Daniell cell is to maintain electrical neutrality in the two half-cells. It achieves this by allowing the migration of ions between the two solutions, completing the electrical circuit. If the salt bridge is removed:
The flow of ions between the half-cells stops.
Positive charge accumulates in the anode compartment (due to Zn → Zn²⁺ + 2e⁻) and negative charge accumulates in the cathode compartment (due to Cu²⁺ + 2e⁻ → Cu).
This charge separation creates an opposing potential that stops the flow of electrons, and the cell ceases to function almost immediately.
2. Describe the working principle of a Daniell cell by stating the reactions occurring at the anode and cathode.
The Daniell cell works on the principle of converting chemical energy into electrical energy through a spontaneous redox reaction. It consists of two half-cells:
At the Anode (Negative Electrode): The zinc electrode undergoes oxidation. Zinc atoms lose electrons and enter the solution as zinc ions. The reaction is: Zn(s) → Zn²⁺(aq) + 2e⁻.
At the Cathode (Positive Electrode): The copper electrode undergoes reduction. Copper ions from the copper sulphate solution gain electrons and deposit as solid copper. The reaction is: Cu²⁺(aq) + 2e⁻ → Cu(s).
The electrons released at the anode travel through the external wire to the cathode, generating an electric current.
3. What are three crucial precautions a student must take while setting up a Daniell cell to ensure an accurate EMF measurement as per the CBSE 2025-26 practical syllabus?
For an accurate EMF measurement in a Daniell cell experiment, students should observe the following precautions:
Clean the Electrodes: The zinc and copper strips must be thoroughly cleaned with sandpaper to remove any oxide or impurity layer, ensuring proper electrochemical activity.
Proper Salt Bridge Preparation: Ensure the salt bridge is completely filled with the electrolyte (like KCl or KNO₃ in agar-agar gel) and has no air bubbles, as they can obstruct the flow of ions.
Use a High-Resistance Voltmeter: A potentiometer or a voltmeter with very high input resistance should be used to measure the EMF. This prevents drawing significant current from the cell, which would otherwise alter the electrode concentrations and give an inaccurate reading.
4. The standard cell potential (E°cell) for a Daniell cell is 1.1V. How is this value calculated from the standard electrode potentials of the half-cells involved?
The standard cell potential (E°cell) is calculated by subtracting the standard reduction potential of the anode from the standard reduction potential of the cathode. The formula is: E°cell = E°cathode - E°anode.
For a Daniell cell under standard conditions (1M concentration, 298K):
The standard reduction potential of the copper half-cell (cathode) is E°(Cu²⁺/Cu) = +0.34 V.
The standard reduction potential of the zinc half-cell (anode) is E°(Zn²⁺/Zn) = -0.76 V.
Therefore, E°cell = (+0.34 V) - (-0.76 V) = 0.34 V + 0.76 V = 1.10 V.
5. Explain the effect of applying an external opposing potential (E_ext) on a Daniell cell in three different scenarios: E_ext < 1.1V, E_ext > 1.1V, and E_ext = 1.1V.
The behaviour of a Daniell cell changes based on the magnitude of the external opposing potential (E_ext) applied:
When E_ext < 1.1 V: The cell functions as a normal galvanic cell. Electrons flow from the zinc electrode to the copper electrode, and current flows from copper to zinc. Zinc dissolves at the anode, and copper deposits at the cathode.
When E_ext = 1.1 V: The external potential exactly balances the cell potential. There is no net flow of electrons or current, and the chemical reaction stops. The cell is in a state of equilibrium.
When E_ext > 1.1 V: The external potential overcomes the cell's potential, forcing the cell to behave like an electrolytic cell. The direction of the current reverses. Electrons flow from the copper electrode to the zinc electrode. Copper dissolves at the copper electrode, and zinc gets deposited at the zinc electrode.
6. How would the EMF of a Daniell cell be affected if the concentration of Zn²⁺ ions is increased? Justify your answer using the Nernst equation.
If the concentration of Zn²⁺ ions is increased, the EMF of the Daniell cell will decrease. This can be explained using the Nernst equation for the cell:
E_cell = E°cell - (RT/nF) ln([Zn²⁺]/[Cu²⁺])
Here, [Zn²⁺] is the concentration of the product ion in the overall reaction (Zn + Cu²⁺ → Zn²⁺ + Cu). According to Le Chatelier's principle and the Nernst equation, increasing the concentration of a product shifts the equilibrium to the left, favouring the reverse reaction. This opposes the forward electromotive force, thus reducing the overall cell potential (E_cell).
7. Write the standard cell notation for a Daniell cell and explain what each part of the notation represents.
The standard cell notation for a Daniell cell is:
Zn(s) | Zn²⁺(aq, 1M) || Cu²⁺(aq, 1M) | Cu(s)
The components of this notation represent:
Zn(s) | Zn²⁺(aq, 1M): This represents the anode half-cell. 'Zn(s)' is the solid zinc electrode, and 'Zn²⁺(aq, 1M)' is the zinc sulphate electrolyte at 1M concentration. The single vertical line '|' denotes a phase boundary between the solid electrode and the aqueous solution.
||: The double vertical line represents the salt bridge, which connects the two half-cells and allows ion migration.
Cu²⁺(aq, 1M) | Cu(s): This represents the cathode half-cell. 'Cu²⁺(aq, 1M)' is the copper sulphate electrolyte at 1M concentration, and 'Cu(s)' is the solid copper electrode. The single vertical line '|' again indicates a phase boundary.
























