Why Are Polysaccharides Essential for Living Organisms?
Polysaccharides are some of the most important biomolecules on Earth, serving critical roles in both plants and animals. Whether you are curious about polysaccharide definition and examples, want to find out is cellulose a polysaccharide, or simply wish to explore examples of polysaccharides, this article will guide you through everything you need to know. Let us analyse their structure, types, and polysaccharide function in organisms, and learn how they impact our everyday lives.
What are Polysaccharides?
A polysaccharide is a large carbohydrate molecule formed by the linkage of many smaller sugar units called monosaccharides. These linked monosaccharides can create straight chains (linear) or branching structures. Since they contain multiple repeating sugar units, polysaccharides are sometimes called complex carbohydrates.
Key Features of Polysaccharides
Non-sweet and generally insoluble: Unlike simple sugars, most polysaccharides do not taste sweet and do not dissolve readily in water.
High molecular weight: They consist of many monosaccharides joined by glycosidic bonds, resulting in a large molecular mass.
Hydrophobic nature: Water usually cannot penetrate these large, often tightly bound molecules, making them hydrophobic.
Osmotically inactive: Because they are large, they do not create significant osmotic pressure within cells, which makes them ideal for storage polysaccharides.
Composed of carbon, hydrogen, and oxygen: The hydrogen-to-oxygen ratio typically remains at 2:1, a hallmark of carbohydrates.
Types of Polysaccharides
Polysaccharides are broadly classified as homopolysaccharides and heteropolysaccharides, based on the nature of their monosaccharide units.
1. Homopolysaccharides
Homopolysaccharides are composed of identical monosaccharide units repeated throughout the chain.
Starch
Found largely in seeds, fruits, and plant storage organs.
Formed by the condensation of two components: amylose (linear) and amylopectin (branched).
A prime example of storage polysaccharides in plants.
Cellulose
When people ask, “Is cellulose a polysaccharide?” the answer is an emphatic yes.
Forms the main component of plant cell walls, providing structural support.
Composed of β-glycosidic linkages that form long, rigid chains.
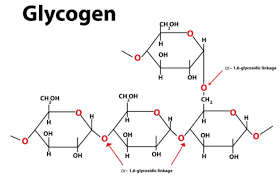
Glycogen
If you have ever wondered, “Is glycogen a polysaccharide?” – it certainly is!
Known as the major storage polysaccharides in animals and fungi.
Highly branched structure, stored primarily in liver and muscle tissues in animals.
Inulin
Made up of multiple fructofuranose units.
Commonly found in plants like dahlia and artichoke tubers.
2. Heteropolysaccharides
Heteropolysaccharides contain different types of monosaccharides in their chains.
Hyaluronic Acid
Composed of D-glucuronic acid and N-acetyl-glucosamine.
Found in connective tissues, skin, and involved in tissue hydration and lubrication.
Heparin
Made of D-glucuronic acid, L-iduronic acid, and N-sulfo-D-glucosamine.
Acts as an anticoagulant in blood and is abundant in mast cells.
Chondroitin-4-Sulfate
Contains D-glucuronic acid and N-acetyl-D-galactosamine-4-O-sulfate.
Present in cartilage, aiding in flexibility and shock absorption.
Gamma Globulin
Includes N-acetyl-hexosamine, D-mannose, D-galactose.
Found in blood and plays a role in immune functions.
Polysaccharide Function
Wondering how these large biomolecules impact living systems? Let’s explore their main roles:
Energy Storage
Storage polysaccharides like starch and glycogen act as energy reservoirs. In plants, starch accumulates in seeds and tubers, while animals synthesise glycogen in muscle and liver.
Structural Support
The question “Is cellulose a polysaccharide?” comes with the crucial role it plays in plant cell walls, giving rigidity and shape.
In insects and fungi, chitin (another polysaccharide) provides structural integrity to their exoskeletons or cell walls.
Cellular Communication
Polysaccharides often bond with lipids (forming glycolipids) and proteins (glycoproteins), enabling cell-to-cell signalling and recognition.
Protection & Lubrication
Hyaluronic acid contributes to cushioning and lubrication in joints.
Heparin helps prevent clotting, ensuring smooth blood flow.
Maintaining Concentration Gradients
Large polysaccharides are osmotically inactive, which aids cells in regulating water and solute levels.
Polysaccharides in Daily Life and Beyond
In addition to their well-known roles, polysaccharides have diverse applications:
Food Industry: Pectin (found in fruit cell walls) is used as a gelling agent in jams, while cellulose derivatives thicken sauces.
Pharmaceuticals: Exopolysaccharides produced by certain bacteria help stabilise formulations in drugs and vaccines.
Eco-Friendly Materials: Research is ongoing into converting cellulose and chitin into biodegradable plastics and fibres.
Quick Quiz: Test Your Polysaccharide Knowledge
True/False: Glycogen is a polysaccharide stored in the liver of animals.
Multiple Choice: Which of the following is a heteropolysaccharide?
a) Starch
b) Cellulose
c) Heparin
d) Glycogen
Fill in the Blank: __________ is found in plant cell walls and provides structural support.
True/False: Hyaluronic acid is composed solely of glucose molecules.
Multiple Choice: Which of these is known as a storage polysaccharide in plants?
a) Inulin
b) Starch
c) Gamma globulin
d) Chondroitin-4-sulfate
Check your answers below
True – Glycogen is indeed a polysaccharide stored in animal liver and muscles.
(c) Heparin – This is a classic example of a heteropolysaccharide.
Cellulose – It is a structural polysaccharide found in plant cell walls.
False – Hyaluronic acid contains D-glucuronic acid and N-acetyl-glucosamine, not just glucose.
(b) Starch – Starch is the main storage polysaccharide in plants.


FAQs on Polysaccharides in Biology: Structure, Types, and Functions
1. What is a polysaccharide and what are some common examples?
A polysaccharide is a large carbohydrate molecule, also known as a complex carbohydrate, formed by the linking of many smaller sugar units called monosaccharides. Key examples found in living organisms include:
- Starch: The primary energy storage molecule in plants.
- Glycogen: The main form of energy storage in animals and fungi.
- Cellulose: A key structural component of plant cell walls.
- Chitin: A structural polysaccharide found in the exoskeletons of arthropods and the cell walls of fungi.
2. How do storage polysaccharides like starch differ from structural polysaccharides like cellulose?
The primary difference lies in their chemical bonds and overall structure, which dictates their function. Storage polysaccharides like starch and glycogen are joined by alpha (α) glycosidic bonds, which form branched, helical structures that are easily broken down by enzymes for energy release. In contrast, structural polysaccharides like cellulose are joined by beta (β) glycosidic bonds, forming long, straight chains that pack together into strong, rigid fibres, providing structural support.
3. Why are polysaccharides not sweet to taste like simple sugars?
Polysaccharides do not taste sweet because their large and complex molecular structure prevents them from binding correctly to the sweet taste receptors on our tongue. These receptors are specifically shaped to interact with small, simple sugar molecules like glucose or fructose. The sheer size of polysaccharides like starch or cellulose means they cannot fit into these receptor sites to trigger a sweet taste signal.
4. What is the importance of starch and glycogen as energy reserves in organisms?
Starch (in plants) and glycogen (in animals) are ideal energy reserves because they are large, insoluble molecules. This allows cells to store vast amounts of glucose energy in a compact form without disrupting the cell's osmotic balance. If glucose were stored directly, it would draw too much water into the cell. When energy is needed, these polymers can be efficiently broken down back into glucose.
5. If humans cannot digest cellulose, why is it considered an important part of our diet?
Although humans lack the enzyme (cellulase) to break down cellulose and use it for energy, it is a vital dietary component known as dietary fibre. Its inability to be digested allows it to add bulk to stool, which aids in digestion and promotes regular bowel movements. It also acts as a prebiotic, supporting the health of beneficial gut bacteria essential for a healthy digestive system.
6. What are the types of polysaccharides based on their monomer units?
Polysaccharides are classified into two main types based on the monosaccharides they contain:
- Homopolysaccharides: These are composed of only one type of monosaccharide unit. Common examples are starch, glycogen, and cellulose, all made entirely of glucose.
- Heteropolysaccharides: These are composed of two or more different types of monosaccharide units or their derivatives. Examples include hyaluronic acid in connective tissue and heparin, an anticoagulant.
7. How does the structure of glycogen make it highly suitable for energy storage in animals?
The structure of glycogen is highly branched, significantly more so than starch. This extensive branching creates numerous terminal ends. When an animal needs a rapid burst of energy, enzymes can work on many of these ends simultaneously to release glucose molecules very quickly. This rapid mobilisation of glucose is critical for functions like muscle contraction, which is why glycogen is the primary energy store in animals rather than starch.
8. What are some important applications of polysaccharides in medicine and industry?
Beyond their biological roles, polysaccharides are extremely useful. In medicine, heparin serves as a vital anticoagulant (blood thinner), and dextran is used as a plasma volume expander. In industry, cellulose is the foundation of the paper and textile industries, while starches are widely used as thickeners and adhesives in food production. Alginates derived from seaweed are used as gelling agents in foods and cosmetics.










