What Is the Process and Significance of Osmosis in Biology?
Osmosis is a vital biological process that involves the movement of water molecules from a region of lower solute concentration to a region of higher solute concentration through a semipermeable membrane. This mechanism is fundamental for cell function, nutrient absorption, and maintaining water balance in living organisms. Let's explore the definition, examples, diagrams, and applications of osmosis in everyday life and biology.
Osmosis Definition
Osmosis can be defined as the passive movement of water molecules across a semipermeable membrane from a dilute solution (low solute concentration) to a concentrated solution (high solute concentration). This process continues until the concentration of solutes on both sides of the membrane becomes equal, achieving equilibrium. A semipermeable membrane allows only specific molecules, such as water, to pass through while restricting larger solute particles.
Osmosis Explanation and Importance
Osmosis is essential for the survival of all living cells. In plants, osmosis enables roots to absorb water from the soil, aiding in nutrient transport and turgidity. In animals, osmosis regulates water content in cells and tissues, helping maintain homeostasis. Disruptions in osmosis can result in dehydration or cell swelling, directly impacting health and vital functions.
Osmosis Diagram
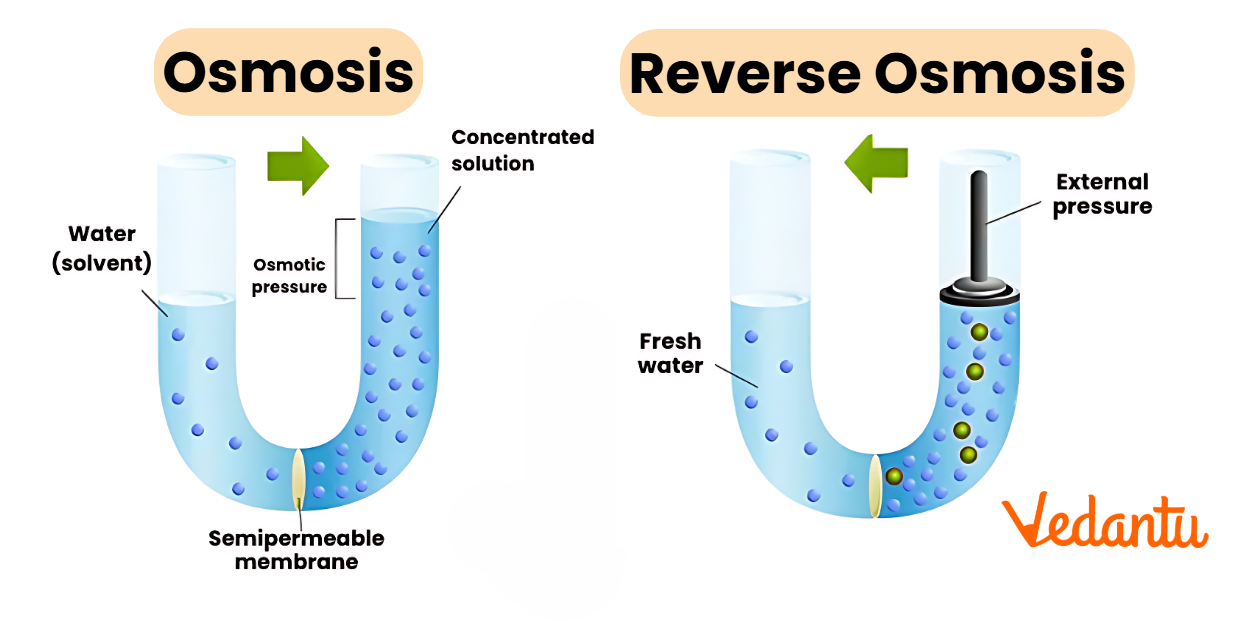
This osmosis diagram visually depicts how water moves across a semipermeable membrane. Water travels from the side with high water concentration (lower solute) to the side with low water concentration (higher solute) until both sides reach balance.
How Does Osmosis Work? Process Step-by-Step
- A semipermeable membrane separates two solutions with different solute concentrations.
- Water molecules move from the side with low solute concentration (high water potential) towards the side with high solute concentration (low water potential).
- The net movement of water continues until concentrations equalize or external pressure stops movement.
This passive process does not require energy. Osmosis differs from simple diffusion as it specifically involves water crossing a membrane.
Osmosis Examples in Daily Life and Biology
Osmosis occurs in many day-to-day scenarios and biological systems:
- Water absorption by plant root hairs from soil.
- Swelling of raisins when soaked in water.
- Movement of water between cells and blood in human kidneys for urine formation.
- The shrinking or swelling of red blood cells in different solutions.
- Opening and closing of plant stomata due to turgor changes caused by osmosis.
For more on how various nutrients are used by the body, see What Do Various Nutrients Do for Our Body.
Types of Solutions Related to Osmosis
Osmosis happens differently depending on the type of surrounding solution:
- Isotonic Solution: Equal solute concentration inside and outside the cell; no net movement of water.
- Hypotonic Solution: Lower solute concentration outside the cell; water moves into the cell, causing swelling.
- Hypertonic Solution: Higher solute concentration outside the cell; water leaves the cell, leading to shrinkage or plasmolysis in plants.
Understanding these solutions helps explain behaviors of cells in different environments, which is critical in medical and plant biology fields. Explore more about Plasmolysis and its significance in plant cells.
Differences Between Osmosis and Diffusion
| Osmosis | Diffusion | Key Feature |
|---|---|---|
| Only water molecules move | Any gas or liquid molecules move | Involves water across a membrane |
| Requires semipermeable membrane | No membrane needed | Membrane specificity |
| Moves from low to high solute area | Moves from high to low concentration | Direction of movement |
While both osmosis and diffusion are forms of passive transport, osmosis specifically refers to water movement. For deeper insights, see the comparison on Diffusion and Osmosis.
Applications and Significance of Osmosis
Osmosis has wide applications beyond biology:
- In Medicine: Used in dialysis to remove waste from the blood in patients with kidney issues.
- In Agriculture: Helps understand water uptake in crops and explains wilting under drought conditions.
- Food Industry: Preserving pickles in brine uses osmosis to draw water out of spoilage-causing bacteria.
- Environmental Science: Osmosis impacts water movement in soil, influencing plant growth and ecosystem health. You can further explore these connections in Effects of Climate Changes.
Understanding osmosis is critical for students studying Cell Theory, human physiology, and plant science. Osmosis also plays a role in food preservation, water purification, and medical treatments like intravenous therapy.
Osmosis in Class 12 Biology and Beyond
Osmosis is a key topic in class 12 biology, featuring in MCQs, theoretical questions, diagrams, and practicals. Students are often required to draw osmosis diagrams, explain its principles, identify osmosis examples, and interpret its significance in experiments. For practice, you can refer to Osmoregulation and Biology Class 9 MCQ: Fundamental Unit of Life.
Quick Osmosis Short Notes
- Osmosis = movement of water through a semipermeable membrane.
- Always passive, never requires energy.
- Essential for water balance in cells, nutrient uptake, and waste removal.
- Key in agriculture, medicine, environment, and food science.
- Common osmosis questions involve diagrams and real-life examples.
For a deeper dive into osmosis notes, explore related concepts like Active Transport and Passive Transport.
Practice: Osmosis Questions and MCQs
- Why does a plant cell burst in pure water but remain stable in a sugar solution?
- Which direction does water travel during osmosis?
- State the difference between osmosis and diffusion.
- What happens to an animal cell in a hypertonic solution?
- Give two real-life osmosis examples from your kitchen.
Remember to revise these for exams, and visit Vedantu for more Food Science or Life Science resources connected to osmosis.
Osmosis is a fundamental concept explaining how water moves in living systems, affecting everything from plant turgor to human health. By mastering osmosis, its diagrams, definitions, examples, and applications, students build a strong foundation for higher studies in biology, medicine, and environmental science. Keep practicing osmosis questions and relate concepts to real-world situations for effective learning.


FAQs on Understanding Osmosis for Students
1. What is osmosis?
Osmosis is the movement of water molecules from a region of high water concentration to a region of low water concentration through a semipermeable membrane.
Key points:
- Osmosis involves only water or another solvent.
- It requires a semipermeable membrane that allows water molecules to pass but blocks solutes.
- It continues until equilibrium is reached between the two sides.
2. What are the main differences between osmosis and diffusion?
Osmosis and diffusion are both passive transport processes, but they differ in key ways.
Main differences:
- Osmosis involves the movement of water across a semipermeable membrane, while diffusion refers to the movement of any molecule (solid, liquid, or gas) from high to low concentration without a membrane required.
- Osmosis occurs only in the case of solvents, primarily water, whereas diffusion applies to both solutes and solvents.
3. What is a semipermeable membrane?
A semipermeable membrane allows certain substances, usually only water molecules, to pass through, while blocking others.
Key features:
- It is essential for processes like osmosis and dialysis.
- Cell membranes are natural examples of semipermeable membranes.
4. Where does osmosis occur in living organisms?
Osmosis occurs in many biological processes within living organisms.
Examples:
- Movement of water into and out of plant root cells
- Regulation of water balance in animal cells
- Absorption of water in the intestines
5. How does osmosis help plants?
Osmosis is vital for plant survival as it helps in the absorption and transport of water.
Functions:
- Water enters root hairs from the soil via osmosis.
- Maintains turgor pressure, supporting plant structure.
- Facilitates transport of nutrients and minerals.
6. What is the difference between endosmosis and exosmosis?
Endosmosis and exosmosis are two types of osmosis based on the direction of water flow.
Differentiation:
- Endosmosis: Entry of water into the cell when placed in a hypotonic solution (cell swells).
- Exosmosis: Exit of water from the cell when placed in a hypertonic solution (cell shrinks).
7. How is osmosis important in the kidneys?
Osmosis is critical in the kidneys for the reabsorption of water during urine formation.
Key processes:
- Helps maintain body fluid balance.
- Concentrates urine by moving water back into the blood from the filtrate in nephron tubules.
- Regulated by antidiuretic hormone (ADH).
8. What is osmotic pressure?
Osmotic pressure is the pressure required to prevent the flow of water across a semipermeable membrane due to osmosis.
Important points:
- It depends on the concentration of solute particles in the solution.
- Higher solute concentration means higher osmotic pressure.
9. Why do animal and plant cells behave differently in hypotonic and hypertonic solutions?
The difference in cell wall presence causes animal and plant cells to behave differently in various solutions.
Effects:
- Plant cells have a cell wall that prevents them from bursting in hypotonic solutions (they become turgid).
- Animal cells lack a cell wall, so they may burst (lysis) in hypotonic and shrink (crenate) in hypertonic solutions.
10. How can osmosis be demonstrated in a laboratory experiment?
Osmosis can be demonstrated using a simple experiment with potato strips and different solutions.
Procedure:
- Place potato strips in water (hypotonic), salt solution (hypertonic), and observe changes.
- In water, potato swells due to endosmosis.
- In salt solution, potato shrinks due to exosmosis.
11. What will happen to a cell if it is kept in a hypertonic solution?
A cell in a hypertonic solution will lose water and shrink due to exosmosis.
Reason:
- Water moves from the cell (higher water concentration) to the surrounding solution (lower water concentration) through the semipermeable membrane.
- This results in the cell becoming flaccid and may even lead to cell death in extreme cases.










